filmov
tv
Kinetics: Using the Integrated Rate Laws and Graphs to Determine the Rate Law
Показать описание
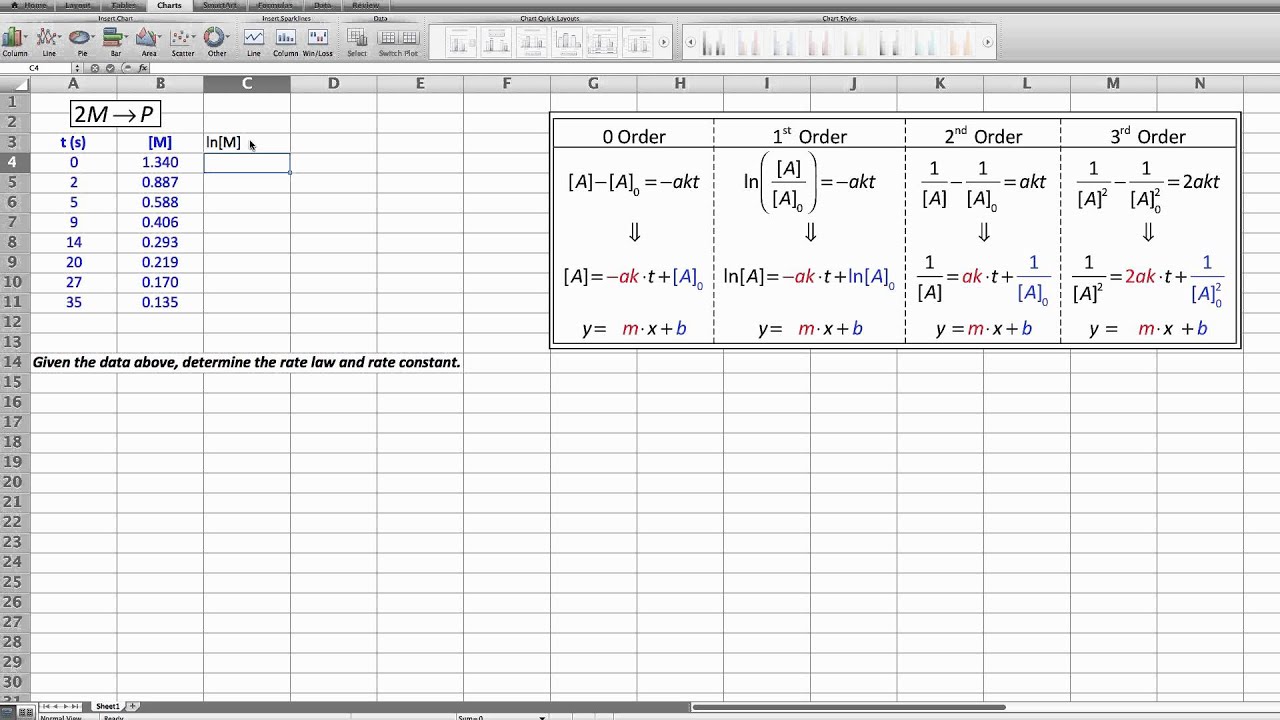
For a unimolecular chemical reaction, a single set of data can reveal the order of reaction. This data is concentration vs. time data and must be graphically analyzed. We start by creating integrated rate laws from the potential rate laws: zero, first, second and third order. This step requires using integral calculus, which is why the four equations that result are called "integrated rate laws".
The integrated rate laws can be put in a linear form in which a function of concentration vs. time could yield a line. "Could" is there because a first order reaction will only yield a linear graph when using the first order integrated rate law. Same for a second order reaction, where it will only be linear when plotted with the proper axis for a second order integrated rate law.
This video walks you through a set of sample data to determine the order of reaction. All four graphs are made so that you can see only one of the four is linear, and that must be the order.
The integrated rate laws can be put in a linear form in which a function of concentration vs. time could yield a line. "Could" is there because a first order reaction will only yield a linear graph when using the first order integrated rate law. Same for a second order reaction, where it will only be linear when plotted with the proper axis for a second order integrated rate law.
This video walks you through a set of sample data to determine the order of reaction. All four graphs are made so that you can see only one of the four is linear, and that must be the order.
Комментарии
 0:09:10
0:09:10
 0:09:55
0:09:55
 0:48:46
0:48:46
 0:08:12
0:08:12
 0:07:13
0:07:13
 0:07:34
0:07:34
 0:10:02
0:10:02
 0:06:43
0:06:43
 0:38:07
0:38:07
 0:34:53
0:34:53
 0:05:54
0:05:54
 0:18:09
0:18:09
 0:04:19
0:04:19
 0:22:18
0:22:18
 0:02:06
0:02:06
 0:25:10
0:25:10
 0:05:03
0:05:03
 0:07:18
0:07:18
 0:18:48
0:18:48
 0:10:49
0:10:49
 0:17:57
0:17:57
 0:09:33
0:09:33
 0:02:06
0:02:06
 0:07:44
0:07:44