filmov
tv
Why do Electronic Orbitals Look Like Clouds

Показать описание
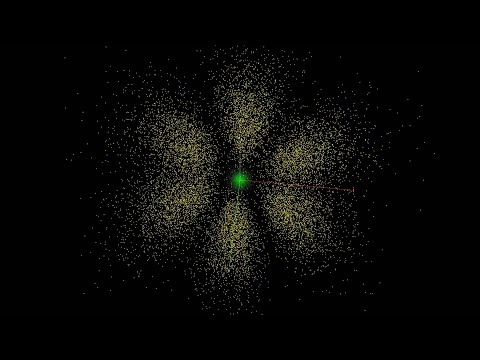
Why do text books make electronic orbitals look like clouds? This is why it makes sense.
Visit us on Instagram:
Three Twentysix Project Leader: Dr Andrew Robertson
Assistant Editor: Purple Saptari
3D animations/production assistant: Es Hiranpakorn
Graphic Design: Maria Sucianto
This video was produced at Kyushu University and supported by JSPS KAKENHI Grant Number JP21K02904. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of Kyushu University, JSPS or MEXT.
Visit us on Instagram:
Three Twentysix Project Leader: Dr Andrew Robertson
Assistant Editor: Purple Saptari
3D animations/production assistant: Es Hiranpakorn
Graphic Design: Maria Sucianto
This video was produced at Kyushu University and supported by JSPS KAKENHI Grant Number JP21K02904. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of Kyushu University, JSPS or MEXT.
Why do Electronic Orbitals Look Like Clouds
What ARE atomic orbitals?
Orbitals: Crash Course Chemistry #25
Atomic Orbitals Simply Explained! Inorganic CHEM - 1.12
what does a dyz orbital look like?
Atomic Orbitals, Visualized Dynamically
Atomic orbitals 3D
Atomic Orbitals
CHE_106_Practice_Exam_4_Part_3
What are Shells, Subshells, and Orbitals? | Chemistry
what does a dz2 orbital look link?
atomic orbitals #orbitals#atom#shorts #youtubeshorts
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Have you ever seen an atom?
Shapes of atomic orbitals (s,p, d)
Atomic orbitals explained #shorts #science
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
How Small is an Atom?
Orbital - Chem Definition
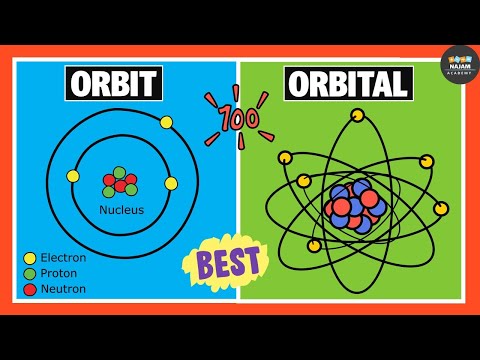
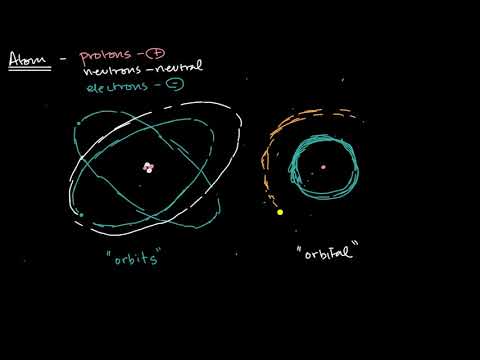
Difference Between Orbits and Orbitals | Chemistry
Shells, subshells and orbitals #chemistrywithsoumya #orbitals #subshells #orbits #shells
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
f-orbital shape of f-orbital
Комментарии
 0:01:00
0:01:00
 0:21:34
0:21:34
 0:10:52
0:10:52
 0:05:56
0:05:56
 0:00:05
0:00:05
 0:08:39
0:08:39
 0:05:50
0:05:50
 0:02:50
0:02:50
 0:16:42
0:16:42
 0:06:00
0:06:00
 0:00:05
0:00:05
 0:00:15
0:00:15
 0:11:19
0:11:19
 0:08:42
0:08:42
 0:02:32
0:02:32
 0:00:33
0:00:33
 0:00:16
0:00:16
 0:09:23
0:09:23
 0:04:52
0:04:52
 0:02:45
0:02:45
 0:08:11
0:08:11
 0:00:59
0:00:59
 0:09:41
0:09:41
 0:00:16
0:00:16