filmov
tv
Western Blot Protocol

Показать описание
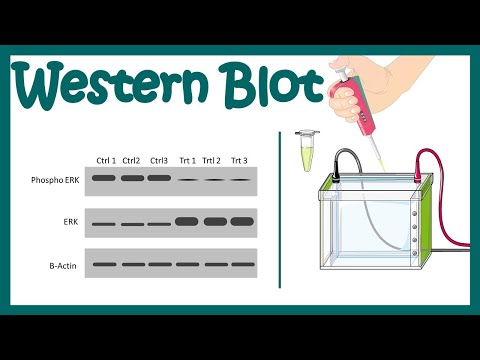
Looking for a way to visualize a protein from cells or tissue samples? Maybe using... the dreaded Western Blot? Well fear not! Meghan from Addgene is here to make sure you'll be running, transferring, and chemiluminescing like a pro by the time you finish this video.
___________________________________________________________________________
00:00 - Intro
00:47 - Materials
02:00 - Preparing Samples
03:57 - Preparing the Gel
07:21 - Using the Transfer Apparatus
10:20 - Primary Antibody Incubation
12:09 - Secondary Antibody Incubation
13:00 - Prepare the Detection Reagent
___________________________________________________________________________
Credits:
Featuring: Meghan Rego
Written By: Meghan Rego, Rachel Leeson, & Quintin Marcelino
Directed, Animated, & Edited by: Quintin Marcelino
Videography by: Quintin Marcelino
Sound Design by: Quintin Marcelino
Designs by: Jason Snair
Addition Designs by: Quintin Marcelino
Music by: Anno Domini Beats & Bail Bonds, courtesy Youtube Audio Library.
Disclaimer: The SOP presented here has been designed by the Addgene nonprofit plasmid repository and is being shared outside of Addgene for informational purposes only. If you choose to reuse or repurpose this SOP in another location, please note that you do so at your own risk; you should ensure that any local guidance is also adhered to. None of the authors, contributors, administrators, or anyone else associated with Addgene, can be held responsible for your use of the information contained in or linked to from these web pages.
___________________________________________________________________________
00:00 - Intro
00:47 - Materials
02:00 - Preparing Samples
03:57 - Preparing the Gel
07:21 - Using the Transfer Apparatus
10:20 - Primary Antibody Incubation
12:09 - Secondary Antibody Incubation
13:00 - Prepare the Detection Reagent
___________________________________________________________________________
Credits:
Featuring: Meghan Rego
Written By: Meghan Rego, Rachel Leeson, & Quintin Marcelino
Directed, Animated, & Edited by: Quintin Marcelino
Videography by: Quintin Marcelino
Sound Design by: Quintin Marcelino
Designs by: Jason Snair
Addition Designs by: Quintin Marcelino
Music by: Anno Domini Beats & Bail Bonds, courtesy Youtube Audio Library.
Disclaimer: The SOP presented here has been designed by the Addgene nonprofit plasmid repository and is being shared outside of Addgene for informational purposes only. If you choose to reuse or repurpose this SOP in another location, please note that you do so at your own risk; you should ensure that any local guidance is also adhered to. None of the authors, contributors, administrators, or anyone else associated with Addgene, can be held responsible for your use of the information contained in or linked to from these web pages.
Комментарии
 0:07:53
0:07:53
 0:09:38
0:09:38
 0:12:40
0:12:40
 0:14:41
0:14:41
 0:08:17
0:08:17
 0:06:32
0:06:32
 0:35:56
0:35:56
 0:03:13
0:03:13
 0:15:01
0:15:01
 0:16:28
0:16:28
 0:10:26
0:10:26
 0:50:05
0:50:05
 0:04:23
0:04:23
 0:03:49
0:03:49
 0:11:46
0:11:46
 0:09:57
0:09:57
 0:02:34
0:02:34
 0:01:56
0:01:56
 0:03:55
0:03:55
 0:10:10
0:10:10
 0:02:01
0:02:01
 0:02:11
0:02:11
 0:31:35
0:31:35
 0:18:00
0:18:00