filmov
tv
1. Properties of WATER | Biochemistry | Notes and Practice Questions in description

Показать описание
Complete Notes and Practice Question
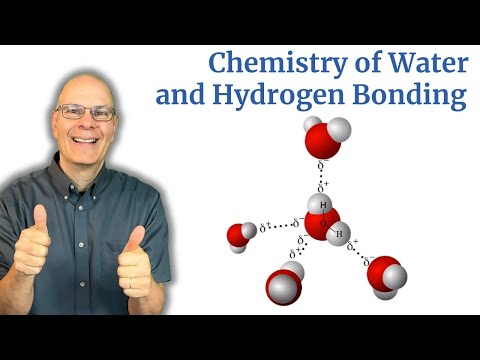
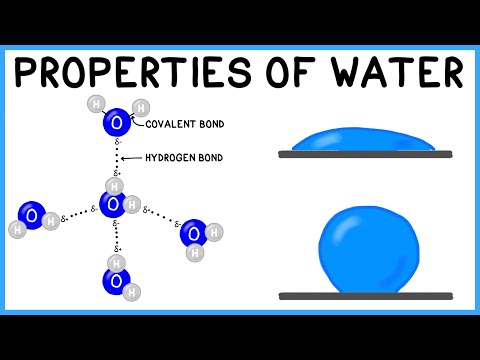
A water molecule has 2 hydrogen atoms covalently bonded to one oxygen atom.
Water is a bent molecule
o Its bent because of the 2 lone pair electrons present on the oxygen atom
Water is a polar molecule
o It’s polar due to the presence of oxygen which is highly electronegative. Oxygen attracts the covalently bonded electrons and gets a partial negative charge and hydrogen gets a partial positive charge, leading to the formation of a dipole.
Water forms intermolecular hydrogen bonds
o Due to partial +ve charge on hydrogen and partial -ve charge on oxygen
o This large difference in charge between hydrogen and oxygen cause force of attraction to act between them (opposite charges) and these forces of attraction are known by a special name “Hydrogen bonds”.
o Hydrogen bonds are not bonds actually they are interactions happening between two atoms with partial opposite charge.
o Similar interactions are also seen when hydrogen bonds with nitrogen and fluorine as both of these are highly electronegative.
Ionic compounds dissolve in water because of interaction between anion and hydrogen atom or cation and oxygen atom
Polar compounds dissolve in water as they have dipole-dipole interactions (Hydrogen bonds)
Polar molecules are hydrophilic and non-polar molecules are hydrophobic.
Ionic compounds are extremely polar and hence they are hydrophilic
Non-polar compounds don’t dissolve in water as water is busy interacting with its own molecule by hydrogen bonds and hence these are called hydrophobic.
As the number of polar groups increases in a compound the solubility of that compound in water increases.
Certain compounds have both polar and non-polar regions in their structure and these are called amphipathic compounds. Eg. Soap molecule and phospholipids (present in the plasma membrane)
If you have any questions, suggestions, or comments please mention them in the comment box.
Please share the content with your friends!
Thanks
A water molecule has 2 hydrogen atoms covalently bonded to one oxygen atom.
Water is a bent molecule
o Its bent because of the 2 lone pair electrons present on the oxygen atom
Water is a polar molecule
o It’s polar due to the presence of oxygen which is highly electronegative. Oxygen attracts the covalently bonded electrons and gets a partial negative charge and hydrogen gets a partial positive charge, leading to the formation of a dipole.
Water forms intermolecular hydrogen bonds
o Due to partial +ve charge on hydrogen and partial -ve charge on oxygen
o This large difference in charge between hydrogen and oxygen cause force of attraction to act between them (opposite charges) and these forces of attraction are known by a special name “Hydrogen bonds”.
o Hydrogen bonds are not bonds actually they are interactions happening between two atoms with partial opposite charge.
o Similar interactions are also seen when hydrogen bonds with nitrogen and fluorine as both of these are highly electronegative.
Ionic compounds dissolve in water because of interaction between anion and hydrogen atom or cation and oxygen atom
Polar compounds dissolve in water as they have dipole-dipole interactions (Hydrogen bonds)
Polar molecules are hydrophilic and non-polar molecules are hydrophobic.
Ionic compounds are extremely polar and hence they are hydrophilic
Non-polar compounds don’t dissolve in water as water is busy interacting with its own molecule by hydrogen bonds and hence these are called hydrophobic.
As the number of polar groups increases in a compound the solubility of that compound in water increases.
Certain compounds have both polar and non-polar regions in their structure and these are called amphipathic compounds. Eg. Soap molecule and phospholipids (present in the plasma membrane)
If you have any questions, suggestions, or comments please mention them in the comment box.
Please share the content with your friends!
Thanks
Комментарии
 0:06:51
0:06:51
 0:04:14
0:04:14
 0:02:45
0:02:45
 0:11:10
0:11:10
 0:04:16
0:04:16
 0:04:26
0:04:26
 0:07:48
0:07:48
 0:12:37
0:12:37
 0:20:01
0:20:01
 0:02:26
0:02:26
 0:11:17
0:11:17
 0:10:37
0:10:37
 0:06:36
0:06:36
 0:04:49
0:04:49
 0:12:51
0:12:51
 0:16:07
0:16:07
 0:10:23
0:10:23
 0:02:13
0:02:13
 0:04:33
0:04:33
 0:08:24
0:08:24
 0:03:58
0:03:58
 0:02:25
0:02:25
 0:10:42
0:10:42
 0:17:33
0:17:33