filmov
tv
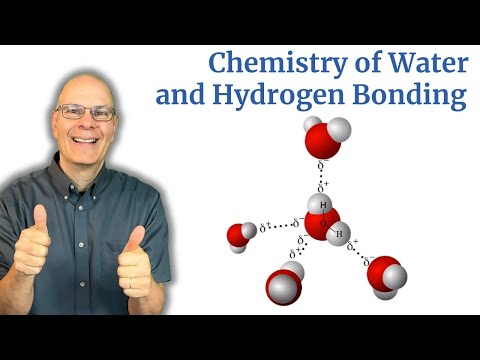
Water: Chemistry and Properties, Part 1

Показать описание
The video covers:
1) How polar covalent bonds develop within a water molecule because of unequal electron sharing
2) How these bonds make water a polar molecule.
3) How water's polarity makes it a "sticky" molecule, resulting in hydrogen bonds that cause molecular cohesion among water molecules.
This video covers content associated with AP Bio Topic 1.1, "Structure of Water and Hydrogen Bonding."
For $29.95 you get a full year's access.
1) How polar covalent bonds develop within a water molecule because of unequal electron sharing
2) How these bonds make water a polar molecule.
3) How water's polarity makes it a "sticky" molecule, resulting in hydrogen bonds that cause molecular cohesion among water molecules.
This video covers content associated with AP Bio Topic 1.1, "Structure of Water and Hydrogen Bonding."
For $29.95 you get a full year's access.
Water: Chemistry and Properties, Part 1
Properties Of Water | Properties of Matter | Chemistry | FuseSchool
Properties of Water
Structure Of Water Molecule - Chemistry Of Water - Properties Of Water - Composition Of Water
Water Chemistry (updated)
The Chemistry of Water | Chemistry Basics
Properties of Water | Hydrogen Bonding in Water | Biology | Biochemistry
Water - Liquid Awesome: Crash Course Biology #2
#sciencefather#researchawards#boosting Water Splitting with RuO2/MoOx/CoNi-LDH Nanocomposite!
How Does Water Bond - Covalent Bonds | Properties of Matter | Chemistry | FuseSchool
Importance of water for life | Chemistry of life | AP Biology | Khan Academy
Water || Structure , Properties, Function #class #jee #biologynotes #biology #physic #biologyclass
Sodium and potassium vs water
Hard & soft water | Environmental Chemistry | Chemistry | FuseSchool
Oxidation of ammonia || pharmacist blogger || #lab #chemistry #laboratory
Filtering sediment from muddy water with a paper towel and some cups
Touching mercury
A satisfying chemical reaction
Electrolysis Of Water | How To Produce Hydrogen From Water | Water Electrolysis #shorts
Hydrophobic Club Moss Spores
| colourful liquid density gradient | layers of liquid in glass |Awesome science experiment
Chemical Reaction 🧫🔥।। Easy science experiment 👨🔬।। #ytshorts #viral #shorts #science...
Acids,Bases and Salt | Science Experiment | By Er Humair Sir | #science #scienceexperiment
Sodium metal is soft and squishy
Комментарии
 0:04:26
0:04:26
 0:04:16
0:04:16
 0:06:51
0:06:51
 0:02:13
0:02:13
 0:12:53
0:12:53
 0:02:25
0:02:25
 0:12:37
0:12:37
 0:11:17
0:11:17
 0:00:43
0:00:43
 0:02:40
0:02:40
 0:08:52
0:08:52
 0:00:05
0:00:05
 0:00:24
0:00:24
 0:04:22
0:04:22
 0:00:11
0:00:11
 0:00:26
0:00:26
 0:00:39
0:00:39
 0:00:19
0:00:19
 0:00:25
0:00:25
 0:00:31
0:00:31
 0:00:16
0:00:16
 0:00:23
0:00:23
 0:00:29
0:00:29
 0:00:38
0:00:38