filmov
tv
Finding Rate Law from Concentration vs Time Data

Показать описание
In this lesson, Mike discusses and strategizes how we should solve the worst kinetic problem scenario:
The one where they give is a bunch of numbers of concentration reactant vs. time and they NEVER tell us the reaction order!
Mike has a a strategy. We shall survive.
The one where they give is a bunch of numbers of concentration reactant vs. time and they NEVER tell us the reaction order!
Mike has a a strategy. We shall survive.
Finding Rate Law from Concentration vs Time Data
Kinetics: Initial Rates and Integrated Rate Laws
How to Find the Rate Law and Rate Constant (k)
Reaction Order Tricks & How to Quickly Find the Rate Law
Solving a Rate Law Using the Initial Rates Method
Aleks Deducing a rate law from the change in concentration over time
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
Concentration-Time Data - Determination of Rate Law and Rate Constant
Working out order from a rate table - tricky example
Chemical Kinetics - Initial Rates Method
DON'T MISS THIS Rate Law and Rate Constant Question
The Rate Law
14.2 Rate Laws | General Chemistry
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
Worked example: Determining a rate law using initial rates data | AP Chemistry | Khan Academy
16.2f Finding half life and rate constant from a graph of concentration versus time
⚗️ Use First-Order Integrated Rate Law to Determine Concentration (Question 1)
Determining Rate Laws from Experimental Data
Rate Law Problems
CH12Q3 Rate Laws and Concentration Changes
14.3 Concentrations and the Rate Law Example #1
Rate law calculations when one reactant is not held constant
R2.2.1 - How do we calculate the rate of reaction from a time-concentration graph?
Calculating Concentration after a Period of Time
Комментарии
 0:22:10
0:22:10
 0:09:10
0:09:10
 0:03:42
0:03:42
 0:01:58
0:01:58
 0:10:49
0:10:49
 0:08:45
0:08:45
 0:18:48
0:18:48
 0:05:41
0:05:41
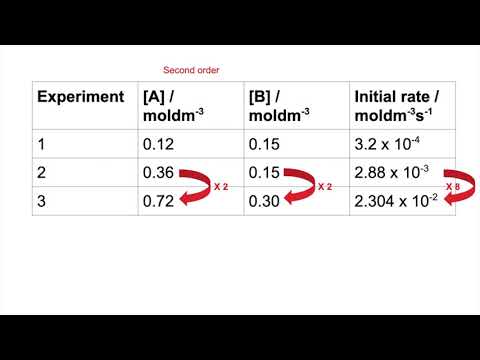 0:02:46
0:02:46
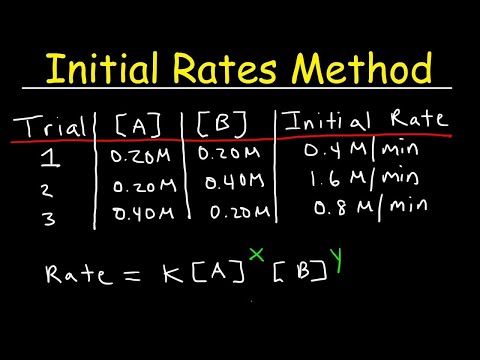 0:34:53
0:34:53
 0:03:46
0:03:46
 0:08:44
0:08:44
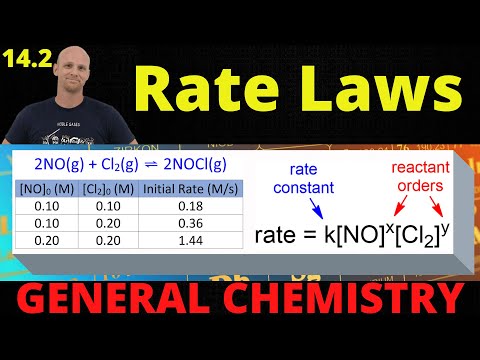 0:25:16
0:25:16
 0:48:46
0:48:46
 0:12:28
0:12:28
 0:04:21
0:04:21
 0:03:59
0:03:59
 0:21:17
0:21:17
 0:18:54
0:18:54
 0:02:04
0:02:04
 0:07:50
0:07:50
 0:12:43
0:12:43
 0:03:18
0:03:18
 0:04:28
0:04:28