filmov
tv
14.2/S2.2 .16 Lewis, hybridization (sp3,sp2,sp) , shapes and angles [HL IB Chemistry]
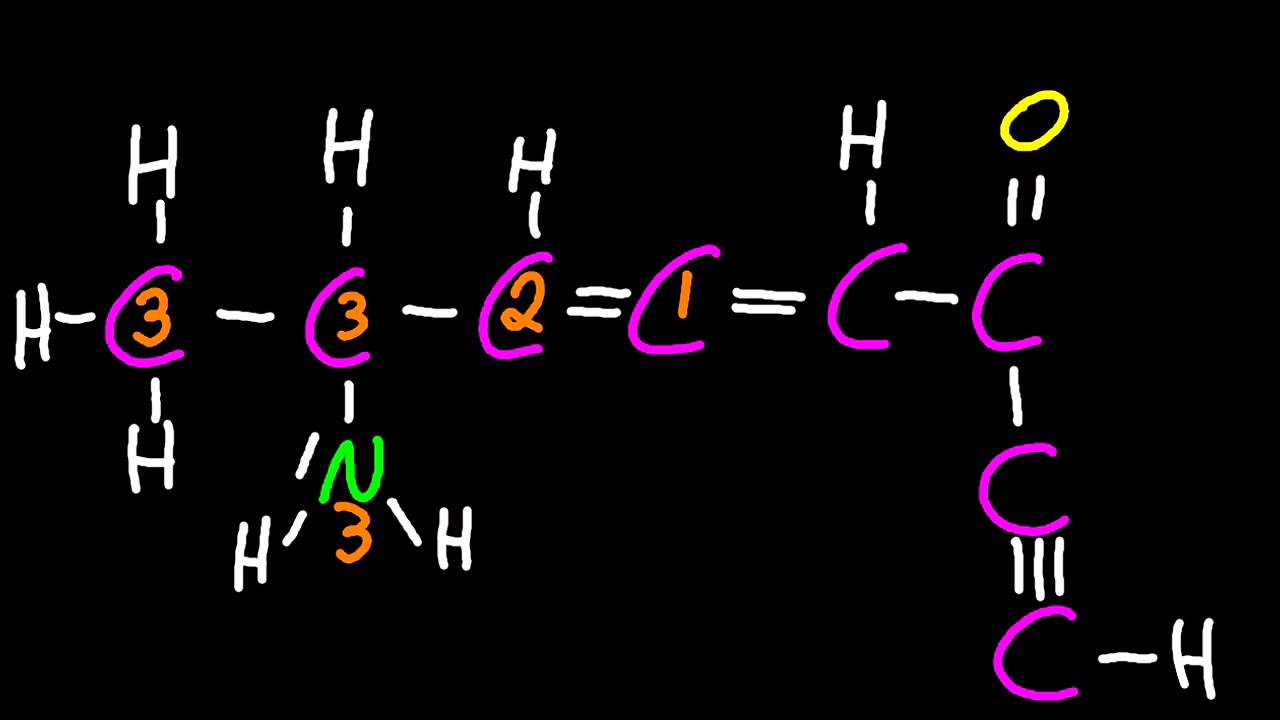
Показать описание
Identify and explain the relationships between Lewis structures, molecular shapes and types of hybridization (sp, sp2 and sp3). If your teacher taught that single bonds means sp3, double means sp2 and triple is sp, break the news that they are wrong!
4 charge centers = sp3, 109.5 / 107 / 105 degree bond angles, 4 sigma
3 charge centers = sp2, 120 degree bond angle, 3 sigma, 1 pi (normally)
2 charge centers = sp, 180 degree bond angle, 2 sigma, 2 pi
4 charge centers = sp3, 109.5 / 107 / 105 degree bond angles, 4 sigma
3 charge centers = sp2, 120 degree bond angle, 3 sigma, 1 pi (normally)
2 charge centers = sp, 180 degree bond angle, 2 sigma, 2 pi
IB Chemistry Topic 14.2 Hybridisation sp sp2 sp3
14.2/S2.2.15 Explain hybridization as mixing of orbitals making new orbitals [HL IB Chemistry]
Topic 14.2 - Hybridization
9.3 Hybridization | General Chemistry
What is Hybridisation ANYWAY?! (IB Chemistry S2.2)
Hybridization - A Simple and Helpful Explanation | IB Chemistry HL 14.2
Bonding Part 9: Hybridization 'a'
Hybridization and VSEPR Theory
MCAT Chemistry: Your VSEPR Theory Study Guide
14. Valence Bond Theory and Hybridization
Hybrid Orbitals 9-1
Chemical Bonding | Hybridization in carbon (sp3, sp2 and sp) | lec 11 | Part 2 | Dr Rizwana
Drawing Molecular Orbital Diagrams
Sp Hybridization Organic Chemistry
Hybridization sp3d examples
General Chemistry 1A. Lecture 13. Hybridization Examples and MO Diagram Introduction.
Shape of Molecules| Hybridization Approach| sp, sp2 and sp3 Hybridization
Hybridization
Chemistry Chemical Bonding part 23 (sp2 hybridization) CBSE class 11 XI
dsp2- hybridization#hybridisation #hybridization #cbse #class12 #cbs_broadcasting #chemicalbonding
Albert Einstein doing physics | very rare video footage #shorts
Hybridization of CH4
Section 1-Hybridization C4U
Hybridization - SP , Sp2 , Sp3 - with examples
Комментарии
 0:08:08
0:08:08
 0:05:28
0:05:28
 0:08:55
0:08:55
 0:16:52
0:16:52
 0:17:14
0:17:14
 0:18:33
0:18:33
 0:14:46
0:14:46
 0:19:32
0:19:32
 0:22:24
0:22:24
 0:56:46
0:56:46
 0:11:13
0:11:13
 0:15:35
0:15:35
 0:11:05
0:11:05
 0:06:38
0:06:38
 0:12:23
0:12:23
 0:46:00
0:46:00
 0:28:19
0:28:19
 0:57:19
0:57:19
 0:05:55
0:05:55
 0:08:19
0:08:19
 0:00:13
0:00:13
 0:03:29
0:03:29
 0:20:47
0:20:47
 0:10:16
0:10:16