filmov
tv
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture (Part 2)
Показать описание
Follow us:
Q1. Write the nuclear equation for each decay process.
(a) beta decay in Bk-249
(b) positron emission in K-40
(c) electron capture in I-111
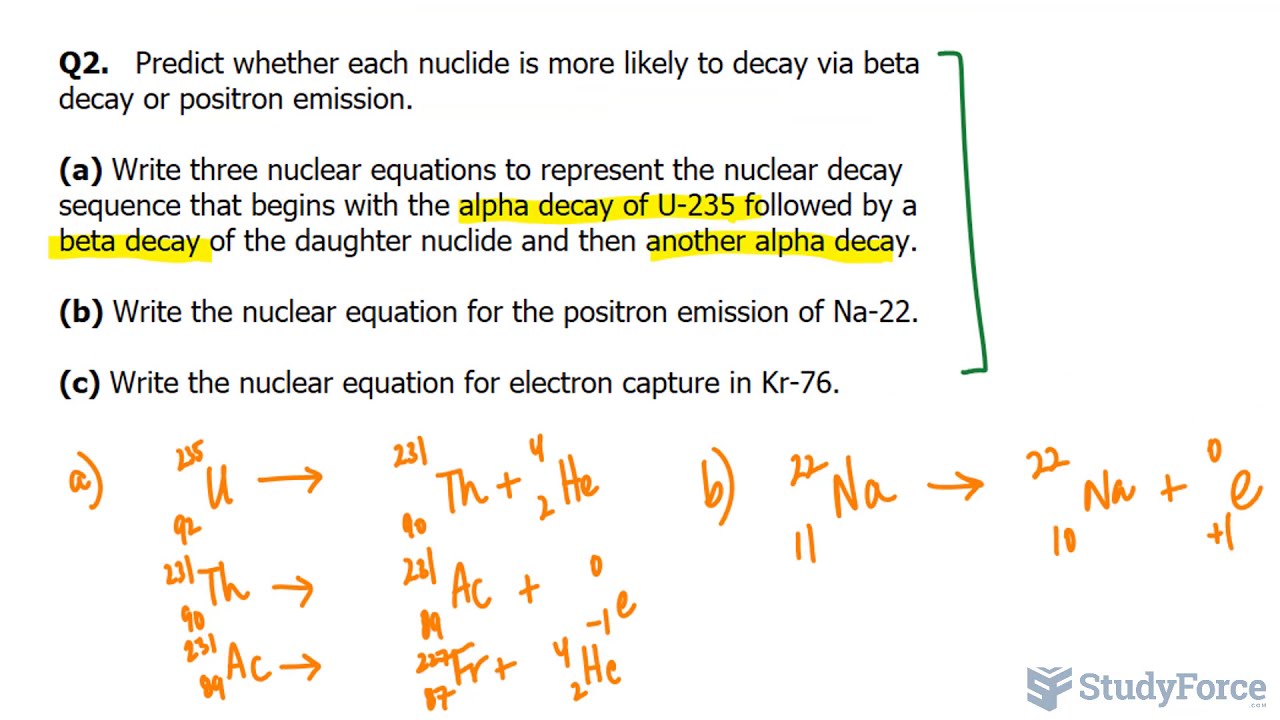
Q2. Predict whether each nuclide is more likely to decay via beta decay or positron emission.
(a) Write three nuclear equations to represent the nuclear decay sequence that begins with the alpha decay of U-235 followed by a beta decay of the daughter nuclide and then another alpha decay.
(b) Write the nuclear equation for the positron emission of Na-22.
(c) Write the nuclear equation for electron capture in Kr-76.
(a) In beta decay, the atomic number increases by 1 and the mass number remains unchanged. The daughter nuclide is element number 98, californium.
(b) In positron emission, the atomic number decreases by 1 and the mass number remains unchanged. The daughter nuclide is element number 18, argon.
(c) In electron capture, the atomic number decreases by 1, and the mass number remains unchanged. The daughter nuclide is element number 52, tellurium.
Writing Beta Decay Nuclear Equations
Writing nuclear equations for alpha, beta, and gamma decay | Chemistry | Khan Academy
GCSE Physics - Nuclear Decay Equations #34
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture
Writing an Equation for Beta Decay
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture (Part 1)
Writing nuclear equations for Beta decay solutions
How To Balance Nuclear Equations In Chemistry
Writing Alpha Decay Nuclear Equations
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture (Part 2)
Writing Positron Decay Nuclear Equations
How To Write Nuclear Equations For Beta Decay
Writing Nuclear Equations for Alpha Decay
Writing nuclear equations for Alpha decay solutions
4- Nuclear Equation
Writing nuclear decay equations
Writing Nuclear Equations
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture-Practice Problems
writing nuclear equations
Solving Nuclear Equations
Writing nuclear equations for alpha, beta, and gamma decay | Chemistry | Khan Academy
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry
Lecture 4.13 - Writing Nuclear Equations
Nuclear Equation Example: Beta decay
Комментарии
 0:02:29
0:02:29
 0:08:07
0:08:07
 0:04:24
0:04:24
 0:07:53
0:07:53
 0:03:31
0:03:31
 0:03:18
0:03:18
 0:02:41
0:02:41
 0:10:46
0:10:46
 0:02:11
0:02:11
 0:06:11
0:06:11
 0:01:35
0:01:35
 0:12:03
0:12:03
 0:01:48
0:01:48
 0:04:21
0:04:21
 0:10:20
0:10:20
 0:03:54
0:03:54
 0:13:30
0:13:30
 0:14:15
0:14:15
 0:10:27
0:10:27
 0:09:53
0:09:53
 0:08:07
0:08:07
 0:17:06
0:17:06
 0:19:25
0:19:25
 0:01:52
0:01:52