filmov
tv
Writing Nuclear Equations for Alpha Decay

Показать описание
Follow us:
Q1. Write the nuclear equation for the alpha decay of Ra-224.
Q2. Write the nuclear equation for the alpha decay of Po-216.
Begin with the symbol for Ra-224 on the left side of the equation and the symbol for an alpha particle on the right side.
Equalize the sum of the mass numbers and the sum of the atomic numbers on both sides of the equation by writing the appropriate mass number and atomic number for the unknown daughter nuclide.
Refer to the periodic table to deduce the identity of the unknown daughter nuclide from the atomic number and write its symbol. Since the atomic number is 86, the daughter nuclide in this example is radon (Rn).
Writing nuclear equations for alpha, beta, and gamma decay | Chemistry | Khan Academy
Writing Nuclear Equations for Alpha Decay
Writing Alpha Decay Nuclear Equations
GCSE Physics - Nuclear Decay Equations #34
Writing nuclear equations for Alpha decay solutions
Writing Nuclear Equations for Alpha Decay
Writing Beta Decay Nuclear Equations
How To Balance Nuclear Equations In Chemistry
4- Nuclear Equation
Writing an Equation for Alpha Decay
Writing Nuclear Equations for Alpha Decay: Practice Problems (Updated)
Writing an Equation for Beta Decay
Writing Nuclear Equations for Alpha Decay-Practice Problems
Writing nuclear equations for alpha, beta, and gamma decay | Chemistry | Khan Academy
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture
Writing nuclear decay equations
Writing Nuclear Equations for Beta Decay, Positron Emission, and Electron Capture (Part 1)
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry
Writing Positron Decay Nuclear Equations
Solving Nuclear Equations
14.2 - Nuclear Equations and Decay
Writing Nuclear Reactions: Introduction and Alpha example
Writing nuclear equations for Beta decay solutions
Types of Radioactivity and Writing Nuclear Equations
Комментарии
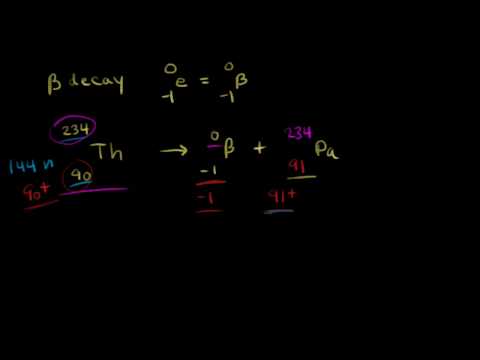 0:08:07
0:08:07
 0:01:48
0:01:48
 0:02:11
0:02:11
 0:04:24
0:04:24
 0:04:21
0:04:21
 0:07:09
0:07:09
 0:02:29
0:02:29
 0:10:46
0:10:46
 0:10:20
0:10:20
 0:05:44
0:05:44
 0:07:12
0:07:12
 0:03:31
0:03:31
 0:06:17
0:06:17
 0:08:07
0:08:07
 0:07:53
0:07:53
 0:03:54
0:03:54
 0:03:18
0:03:18
 0:17:06
0:17:06
 0:01:35
0:01:35
 0:09:53
0:09:53
 0:16:30
0:16:30
 0:04:59
0:04:59
 0:02:41
0:02:41
 0:16:46
0:16:46