filmov
tv
Kinetics and Reaction Rates (AP Chemistry)

Показать описание
Here, we work through an AP Chemistry multiple choice question (MCQ) that focuses on kinetics and reaction rates. Specifically, we look at the method of initial rates, where we look at how the initial concentration of a reactant changes, and see what effect that has on the rate of the reaction. There are a number of different ways to solve a problem like this: we will look at a logic-based approach and then some more mathematical methods. Most importantly, we have to determine whether the reaction is zero order, first order, or second order with respect to the various reactants. This allows us to write a rate law equation. This problem is specifically written for the AP Chemistry test, but it also may be useful for other tests and curriculum such as MCAT, OAT, DAT, SAT, IGCSE, A-Levels, IB, CBSE, JEE, and NEET.
Kinetics and Reaction Rates (AP Chemistry)
Kinetics: Initial Rates and Integrated Rate Laws
Introduction to reaction rates | Kinetics | AP Chemistry | Khan Academy
AP Chem Unit 5 Review - Kinetics in 10 Minutes!
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32
AP Chem Unit 5 Review | Chemical Kinetics in 10 Minutes!
Factors Affecting the Rate of the Reaction - Chemical Kinetics
Reaction Rates & Introduction to Kinetics - AP Chem Unit 5, Topic 1
Chemical Kinetics - Initial Rates Method
14.1 Rate Expressions and the Rate of Reaction | General Chemistry
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
AP® Chemistry Kinetics Questions Free Response
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
AP Chemistry Unit 5 Kinetics Multiple Choice Problem (Rate Law & Orders)
The Rate Law
Factors affecting reaction rates | Kinetics | AP Chemistry | Khan Academy
Reaction Order Tricks & How to Quickly Find the Rate Law
Chemical kinetics Activation energy
An Introduction to Chemical Kinetics
First-order reactions | Kinetics | AP Chemistry | Khan Academy
Collision theory | Kinetics | AP Chemistry | Khan Academy
Rate law and reaction order | Kinetics | AP Chemistry | Khan Academy
AP Chem - Full kinetics review guide
Комментарии
 0:07:18
0:07:18
 0:09:10
0:09:10
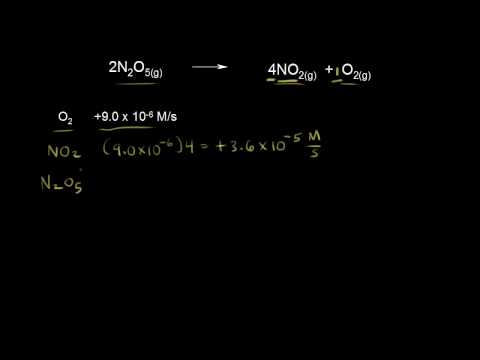 0:09:14
0:09:14
 0:10:09
0:10:09
 0:09:57
0:09:57
 0:10:56
0:10:56
 0:06:14
0:06:14
 0:19:13
0:19:13
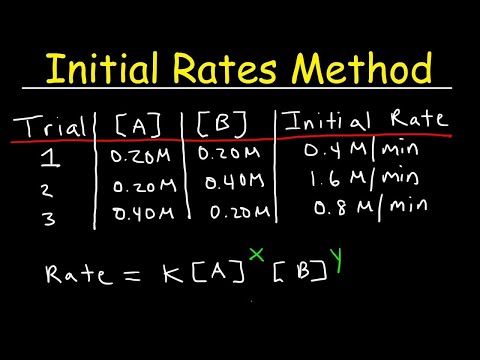 0:34:53
0:34:53
 0:10:39
0:10:39
 0:48:46
0:48:46
 0:08:42
0:08:42
 0:15:09
0:15:09
 0:18:48
0:18:48
 0:02:37
0:02:37
 0:08:44
0:08:44
 0:05:29
0:05:29
 0:01:58
0:01:58
 0:00:08
0:00:08
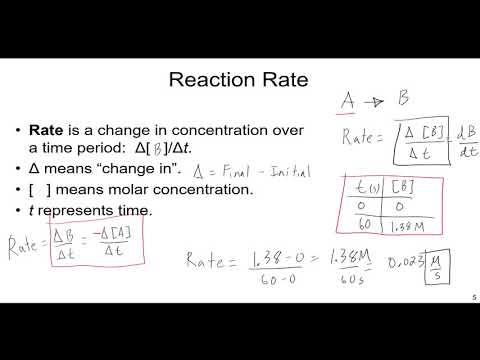 0:25:24
0:25:24
 0:07:44
0:07:44
 0:08:48
0:08:48
 0:10:39
0:10:39
 0:15:12
0:15:12