filmov
tv
Calculating rate constant for 2nd order reaction via integrated rate law.

Показать описание
Question: Based on these data, the rate constant for the reaction is ?
The decomposition of nitrosyl bromide at 10 °CNOBrNO + ½ Br2is second order in NOBr.In one experiment, when the initial concentration of NOBr was 0.347 M, the concentration of NOBr dropped to 5.48×10-2 M after 13.8 seconds had passed.Based on these data, the rate constant for the reaction is _____ M-1 s-1.
------------------------
Answered By:
Jonathan H.
General Biology Tutor for 3+ Years
------------------------
Written Explanation:
Answer is: k= 1.114 M-1s-1
------------------------
About: Wyzant Ask an Expert offers free answers to your toughest academic and professional questions from over 65,000 verified experts. It’s trusted by millions of students each month with the majority of questions receiving an answer within 1 hour of being asked. If you ever need more than just an answer, Wyzant also offers personalized 1-on-1 sessions with experts that will work with you to help you understand whatever you’re trying to learn.
 0:04:36
0:04:36
 0:03:42
0:03:42
 0:12:15
0:12:15
 0:09:10
0:09:10
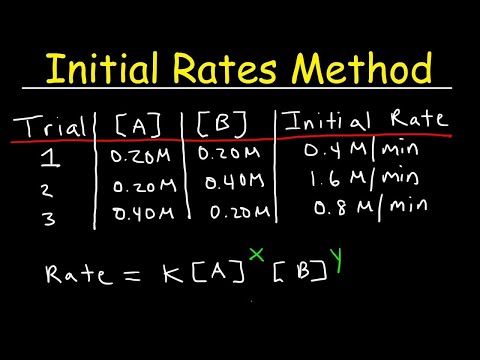 0:34:53
0:34:53
 0:05:51
0:05:51
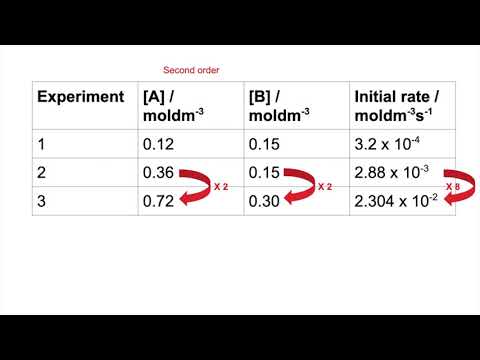 0:02:46
0:02:46
 0:06:15
0:06:15
 3:06:51
3:06:51
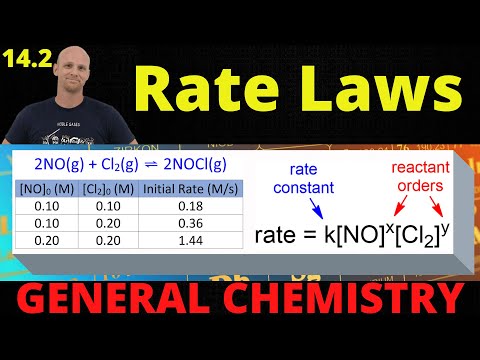 0:25:16
0:25:16
 0:04:35
0:04:35
 0:01:58
0:01:58
 0:01:46
0:01:46
 0:07:18
0:07:18
 0:03:44
0:03:44
 0:10:49
0:10:49
 0:02:42
0:02:42
 0:08:15
0:08:15
 0:18:48
0:18:48
 0:00:59
0:00:59
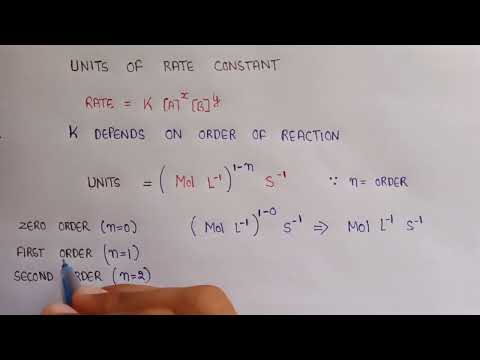 0:03:17
0:03:17
 0:12:43
0:12:43
 0:10:30
0:10:30
 0:25:10
0:25:10