filmov
tv
Hess' Law and Enthalpy Cycles | A-level Chemistry | OCR, AQA, Edexcel

Показать описание
Hess' Law and Enthalpy Cycles in a Snap!
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Hess’ Law
2. Enthalpy Cycles
a. Enthalpy Change of Combustion
b. Enthalpy Change of Formation
Hess’ Law
Germain Hess, a chemist and doctor, proposed Hess’ Law. Hess’ Law states that: The enthalpy change of reaction is independent of the route taken.
Enthalpy Cycles
The enthalpy change of a reaction cannot always be directly measured. We can use enthalpy cycles to help us to indirectly calculate the enthalpy change. An enthalpy cycle is a pictorial representation showing the alternative routes of reaction between reactants and products. Enthalpy cycles are triangular. In each corner there should be the same number of each atom. As well as energy, matter is also conserved. The sides of the triangle are arrows.
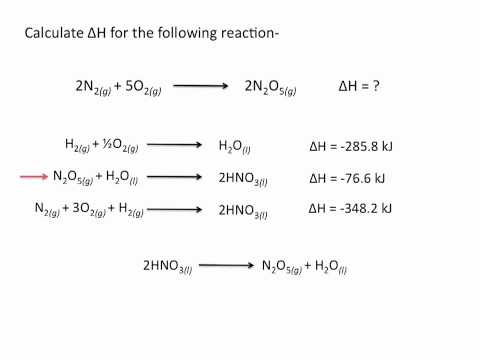
Enthalpy Cycle Example: Combustion
We can use the enthalpy changes of combustion to find the enthalpy change of the reaction. Carbon and Hydrogen can be combusted to form carbon dioxide and water. Benzene can be combusted to form carbon dioxide and water.
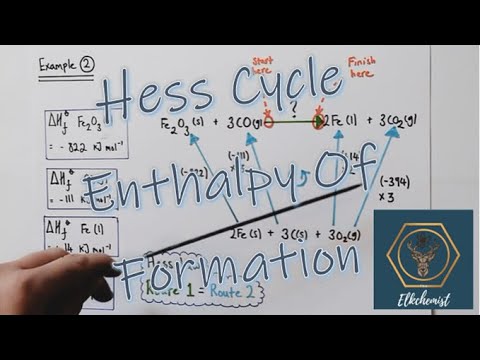
Enthalpy Cycle Example: Formation
We can use the enthalpy changes of formation to find the enthalpy change of the reaction. Nitrogen monoxide and oxygen can be made from nitrogen and oxygen, Nitrodgen dioxide can be formed from nitrogen and oxygen.
Summary
Hess' Law states that the enthalpy change of a reaction is independent of the route taken
Enthalpy Cycles are a pictorial representation of the routes of reaction
a. Triangle shape
b. Chemicals are the corners of the triangle
c. Arrows are the sides
i. These show the direction of the chemical reaction
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Hess’ Law
2. Enthalpy Cycles
a. Enthalpy Change of Combustion
b. Enthalpy Change of Formation
Hess’ Law
Germain Hess, a chemist and doctor, proposed Hess’ Law. Hess’ Law states that: The enthalpy change of reaction is independent of the route taken.
Enthalpy Cycles
The enthalpy change of a reaction cannot always be directly measured. We can use enthalpy cycles to help us to indirectly calculate the enthalpy change. An enthalpy cycle is a pictorial representation showing the alternative routes of reaction between reactants and products. Enthalpy cycles are triangular. In each corner there should be the same number of each atom. As well as energy, matter is also conserved. The sides of the triangle are arrows.
Enthalpy Cycle Example: Combustion
We can use the enthalpy changes of combustion to find the enthalpy change of the reaction. Carbon and Hydrogen can be combusted to form carbon dioxide and water. Benzene can be combusted to form carbon dioxide and water.
Enthalpy Cycle Example: Formation
We can use the enthalpy changes of formation to find the enthalpy change of the reaction. Nitrogen monoxide and oxygen can be made from nitrogen and oxygen, Nitrodgen dioxide can be formed from nitrogen and oxygen.
Summary
Hess' Law states that the enthalpy change of a reaction is independent of the route taken
Enthalpy Cycles are a pictorial representation of the routes of reaction
a. Triangle shape
b. Chemicals are the corners of the triangle
c. Arrows are the sides
i. These show the direction of the chemical reaction
Комментарии
 0:13:46
0:13:46
 0:14:03
0:14:03
 0:04:58
0:04:58
 0:24:15
0:24:15
 0:05:11
0:05:11
 0:07:25
0:07:25
 0:10:05
0:10:05
 0:13:34
0:13:34
 0:11:42
0:11:42
 0:08:39
0:08:39
 0:10:21
0:10:21
 0:11:03
0:11:03
 0:03:11
0:03:11
 0:35:58
0:35:58
 0:05:44
0:05:44
 0:11:23
0:11:23
 0:08:19
0:08:19
 0:24:54
0:24:54
 0:00:59
0:00:59
 0:19:14
0:19:14
 0:11:24
0:11:24
 0:02:32
0:02:32
 0:35:20
0:35:20
 0:04:11
0:04:11