filmov
tv
INTRAMOLECULAR BONDING - COVALENT, IONIC, METALLIC

Показать описание
Bonding occurs because atoms wish to arrange themselves in the most stable patterns possible. Stability is achieved through the completion of the atom’s outermost electron orbits, thus obtaining the octet configuration in the valence shell and satisfying the octet rule. Atoms can do this by joining other atoms via chemical bonds.
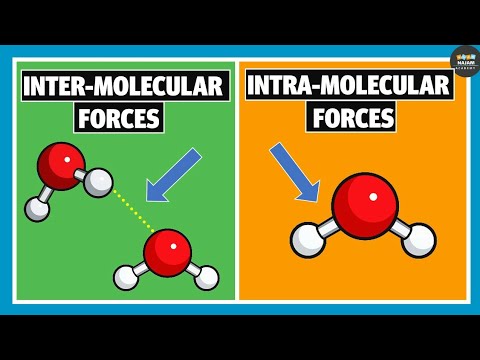
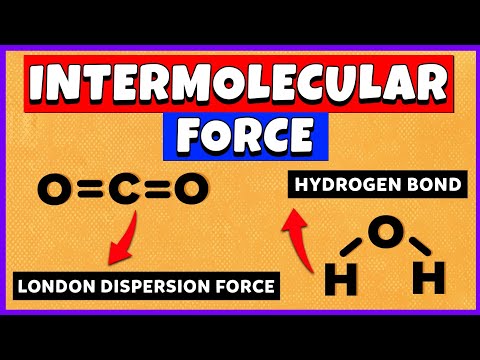
First, let’s differentiate between intramolecular bonds and intermolecular forces! Intramolecular bonds bond atoms to other atoms, creating compounds. In contrast, intermolecular forces attract atoms and molecules to other atoms and molecules.
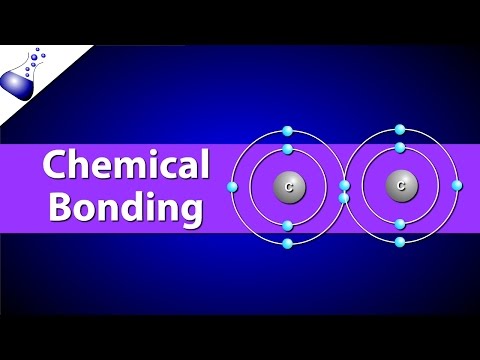
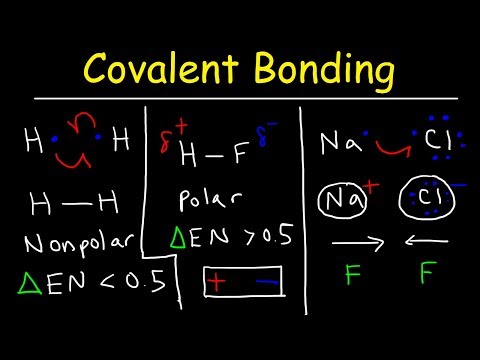
This video will focus on intramolecular bonds – covalent, ionic, and metallic. A covalent bond, also known as a molecular bond, is formed when a pair or pairs of electrons are shared between two atoms to form a covalently-bonded species or a molecular compound. As a result, the pair of shared electrons forms a new molecular orbital extending around the nuclei of both atoms to form a molecule.
Covalent bonds are the most common bond in organic molecules, and you can get compounds with high molecular mass in this way. For example, macromolecules can be linear, branched, or cross-linked. You can also get a crystal network or lattice in which each atom is covalently bonded to its neighbors to form one large molecule. Sometimes you can have different allotropes – different forms of an element in its natural state. For example, graphite and diamonds are both allotropes of carbon.
Covalent bonds can be polar and nonpolar, depending on the electronegativities of the atoms involved. Electronegativity is the tendency of an atom to attract a shared pair of bonded electrons in its combined state. Electropositivity is the opposite. A polar covalent bond is formed between 2 different nonmetal atoms with different electronegativities, which results in them sharing electrons unequally. The bonding electron pair is closer to one of the nuclei, depending on the relative electronegativies of the 2 atoms. So although the overall molecule is neutral, this results in one end of the molecule being slightly negatively charged and the other slightly positively charged, and this charge distribution is denoted by a dipole arrow and a lowercase delta with a charge superscript. Substances with polar covalent bonds have higher melting and boiling points. They are also soluble in polar compounds.
A nonpolar covalent bond is formed between two atoms with the same or very similar electronegativity. They share their electrons equally. These substances tend to exist as gases and rarely as liquids. They have low melting and boiling points and are soluble in nonpolar solvents.
What makes atoms form covalent or ionic bonds? In covalent bonding, the difference between the electronegativities of the 2 atoms is insufficient for an electron transfer to occur and to form ions. The atoms involved have high ionization energies. Ionization energy is the energy needed to remove electrons from a neutral atom, forming a positively-charged ion. The 1st ionization energy of an atom is the energy needed to remove the outermost, highest energy electron when it is neutral and in gas phase. Note how this ionization energy is inversely proportional to atomic size. As you travel right on the periodic table, you have more protons, which attract the electrons in closer to the nucleus and make it harder for them to escape. As you go down, with each row you get a new shell of electrons, and so the valence electrons are further from the nucleus and easier to take away!
Ionic bonds hold atoms together via strong electrostatic attraction between charged ions which differ significantly in electronegativity. As a result, the less electronegative ion transfers electrons to the more electronegative ion. The result is an anion with a negative charge and a cation with a positive charge. These opposite charges attract to form a molecule. As an example, let’s take sodium chloride! Chloride needs an electron to complete its octet. The easiest way for sodium to have a full octet valence shell is to lose it s one valence electron. So sodium donates an electron to chloride. As a result, sodium is now a cation and chloride is now an anion. Note that ionic compounds can dissociate into ions in solution!
The likelihood of an ionic bond forming depends on the radius of the atoms. A larger radius increases the likelihood of ionic bonding by decreasing the ionization energy. Because ionic bonds form between atoms with big differences in electronegativity, they form between a metal and a nonmetal.
Lastly, metallic bonds are formed between metals, metalloids, and alloys. The bond is formed between positively-charged atoms that share electrons. The valence electrons go from one atom to the next, continuously moving throughout the entire space. These free electrons have been described as a “sea of electrons”.
Different types of bonds result in different properties.
First, let’s differentiate between intramolecular bonds and intermolecular forces! Intramolecular bonds bond atoms to other atoms, creating compounds. In contrast, intermolecular forces attract atoms and molecules to other atoms and molecules.
This video will focus on intramolecular bonds – covalent, ionic, and metallic. A covalent bond, also known as a molecular bond, is formed when a pair or pairs of electrons are shared between two atoms to form a covalently-bonded species or a molecular compound. As a result, the pair of shared electrons forms a new molecular orbital extending around the nuclei of both atoms to form a molecule.
Covalent bonds are the most common bond in organic molecules, and you can get compounds with high molecular mass in this way. For example, macromolecules can be linear, branched, or cross-linked. You can also get a crystal network or lattice in which each atom is covalently bonded to its neighbors to form one large molecule. Sometimes you can have different allotropes – different forms of an element in its natural state. For example, graphite and diamonds are both allotropes of carbon.
Covalent bonds can be polar and nonpolar, depending on the electronegativities of the atoms involved. Electronegativity is the tendency of an atom to attract a shared pair of bonded electrons in its combined state. Electropositivity is the opposite. A polar covalent bond is formed between 2 different nonmetal atoms with different electronegativities, which results in them sharing electrons unequally. The bonding electron pair is closer to one of the nuclei, depending on the relative electronegativies of the 2 atoms. So although the overall molecule is neutral, this results in one end of the molecule being slightly negatively charged and the other slightly positively charged, and this charge distribution is denoted by a dipole arrow and a lowercase delta with a charge superscript. Substances with polar covalent bonds have higher melting and boiling points. They are also soluble in polar compounds.
A nonpolar covalent bond is formed between two atoms with the same or very similar electronegativity. They share their electrons equally. These substances tend to exist as gases and rarely as liquids. They have low melting and boiling points and are soluble in nonpolar solvents.
What makes atoms form covalent or ionic bonds? In covalent bonding, the difference between the electronegativities of the 2 atoms is insufficient for an electron transfer to occur and to form ions. The atoms involved have high ionization energies. Ionization energy is the energy needed to remove electrons from a neutral atom, forming a positively-charged ion. The 1st ionization energy of an atom is the energy needed to remove the outermost, highest energy electron when it is neutral and in gas phase. Note how this ionization energy is inversely proportional to atomic size. As you travel right on the periodic table, you have more protons, which attract the electrons in closer to the nucleus and make it harder for them to escape. As you go down, with each row you get a new shell of electrons, and so the valence electrons are further from the nucleus and easier to take away!
Ionic bonds hold atoms together via strong electrostatic attraction between charged ions which differ significantly in electronegativity. As a result, the less electronegative ion transfers electrons to the more electronegative ion. The result is an anion with a negative charge and a cation with a positive charge. These opposite charges attract to form a molecule. As an example, let’s take sodium chloride! Chloride needs an electron to complete its octet. The easiest way for sodium to have a full octet valence shell is to lose it s one valence electron. So sodium donates an electron to chloride. As a result, sodium is now a cation and chloride is now an anion. Note that ionic compounds can dissociate into ions in solution!
The likelihood of an ionic bond forming depends on the radius of the atoms. A larger radius increases the likelihood of ionic bonding by decreasing the ionization energy. Because ionic bonds form between atoms with big differences in electronegativity, they form between a metal and a nonmetal.
Lastly, metallic bonds are formed between metals, metalloids, and alloys. The bond is formed between positively-charged atoms that share electrons. The valence electrons go from one atom to the next, continuously moving throughout the entire space. These free electrons have been described as a “sea of electrons”.
Different types of bonds result in different properties.
Комментарии
 0:06:07
0:06:07
 0:03:33
0:03:33
 0:10:54
0:10:54
 0:11:50
0:11:50
 0:05:36
0:05:36
 0:06:53
0:06:53
 0:02:15
0:02:15
 0:05:33
0:05:33
 0:06:40
0:06:40
 0:01:35
0:01:35
 0:04:12
0:04:12
 0:04:46
0:04:46
 0:10:35
0:10:35
 0:05:37
0:05:37
 0:06:30
0:06:30
 0:08:17
0:08:17
 0:14:32
0:14:32
 0:05:19
0:05:19
 0:07:04
0:07:04
 0:21:16
0:21:16
 0:06:08
0:06:08
 0:08:05
0:08:05
 0:12:14
0:12:14
 0:08:07
0:08:07