filmov
tv
3.4 Limiting Reactant Problems | General Chemistry
Показать описание
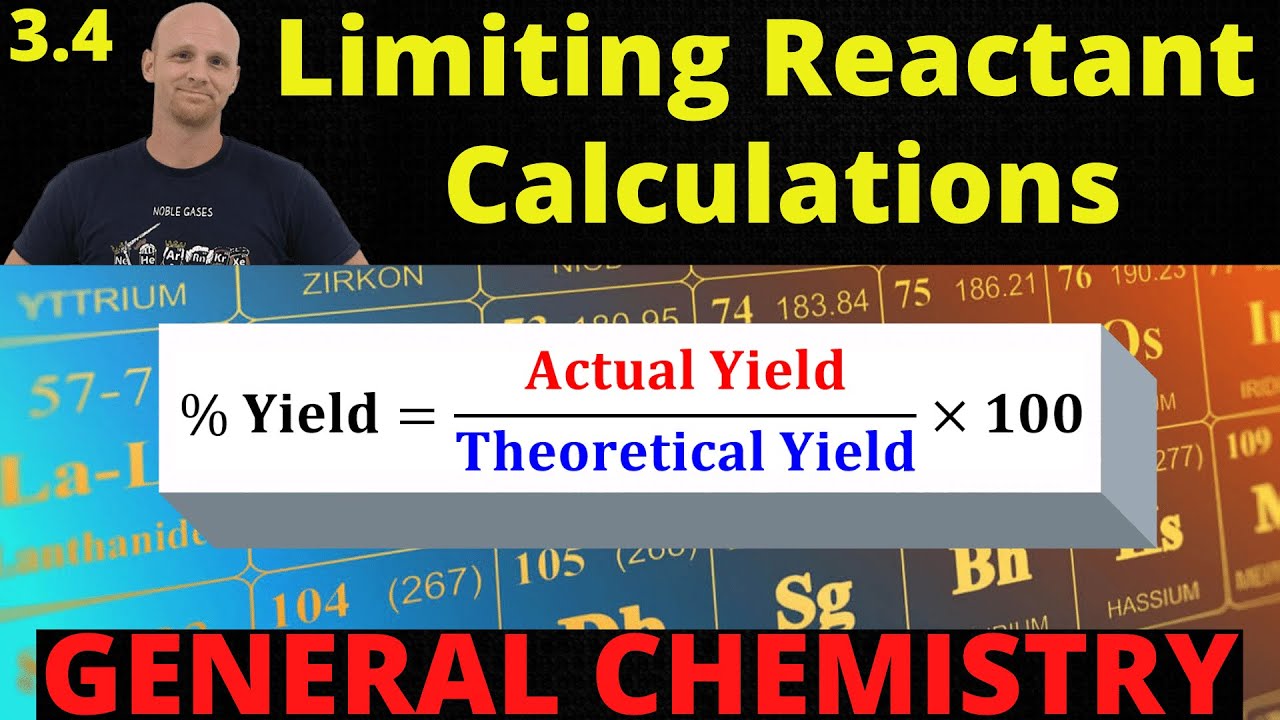
In this lesson Chad shows how to solve Limiting Reactant Problems. This includes how to determine the limiting reagent, how to determine the reagent in excess, and how much of the reagent in excess remains. It also includes how to calculate the theoretical yield, actual yield, and percent yield of a reaction as well.
Chad begins by showing how the coefficients in a balanced chemical reaction provide mole-to-mole ratios that are the basis of Limiting Reactant Calculations. These mole-to-mole ratios can then be used to convert moles of reactants to moles of products. Ultimately, whichever reactant would lead to the production of the least amount of product is the limiting reactant, while the others are the reactants in excess.
Chad begins with a practice limiting reactant problem involving bicycles to provide a more accessible example for students. The lesson then progresses to limiting reactant practice problems that involve starting with the number of moles of the reactants before moving on to limiting reactant problems that involve starting with the masses in grams of the reactants rather than the moles. The lesson concludes with a complex Limiting Reactant problem that includes how to determine the limiting reagent, how to calculate the theoretical yield, how to determine the amount of the reagent in excess that remains, and how to calculate the percent yield.
00:00 Lesson Introduction
00:47 Mole-to-Mole Ratios in Stoichiometry
09:44 Introduction to Limiting Reactant Calculations
13:15 Limiting Reactant Calculations
21:24 Percent Yield Calculations
Chad begins by showing how the coefficients in a balanced chemical reaction provide mole-to-mole ratios that are the basis of Limiting Reactant Calculations. These mole-to-mole ratios can then be used to convert moles of reactants to moles of products. Ultimately, whichever reactant would lead to the production of the least amount of product is the limiting reactant, while the others are the reactants in excess.
Chad begins with a practice limiting reactant problem involving bicycles to provide a more accessible example for students. The lesson then progresses to limiting reactant practice problems that involve starting with the number of moles of the reactants before moving on to limiting reactant problems that involve starting with the masses in grams of the reactants rather than the moles. The lesson concludes with a complex Limiting Reactant problem that includes how to determine the limiting reagent, how to calculate the theoretical yield, how to determine the amount of the reagent in excess that remains, and how to calculate the percent yield.
00:00 Lesson Introduction
00:47 Mole-to-Mole Ratios in Stoichiometry
09:44 Introduction to Limiting Reactant Calculations
13:15 Limiting Reactant Calculations
21:24 Percent Yield Calculations
Комментарии
 0:18:52
0:18:52
 0:05:04
0:05:04
 0:07:16
0:07:16
 0:20:46
0:20:46
 0:10:47
0:10:47
 0:09:21
0:09:21
 0:04:45
0:04:45
 0:04:21
0:04:21
 0:08:52
0:08:52
 0:06:04
0:06:04
 0:05:37
0:05:37
 0:14:05
0:14:05
 0:04:35
0:04:35
 0:08:07
0:08:07
 0:15:53
0:15:53
 0:07:53
0:07:53
 0:03:32
0:03:32
 0:13:32
0:13:32
 0:16:09
0:16:09
 0:16:12
0:16:12
 0:10:04
0:10:04
 0:07:34
0:07:34
 0:01:00
0:01:00
 0:10:07
0:10:07