filmov
tv
Iodine Clock Reaction

Показать описание
You can try an at home version of this experiment using a few things you may have in your bathroom medicine cabinet. In may ways this experiment feels almost like magic.
What you need:
distilled water (tap water will work OK as well)
a couple plastic cups
1000 mg vitamin C tablets
tincture of iodine (2%)
hydrogen peroxide (3%)
liquid laundry starch
What to do:
Make a vitamin C solution by crushing a 1000 mg vitamin C tablet and dissolving it in 2 oz of water. Label this as “vitamin C stock solution”.
Combine 1 tsp of the vitamin C stock solution with 1 tsp of iodine and 2 oz of water. Label this “solution A”.
Prepare “solution B” by adding 2 oz of water to 3 tsp of hydrogen peroxide and 1/2 tsp of liquid starch solution.
Pour solution A into solution B, and pour the resulting solution back into the empty cup to mix them thoroughly. Keep pouring the liquid back and fourth between the cups.
What’s going on?
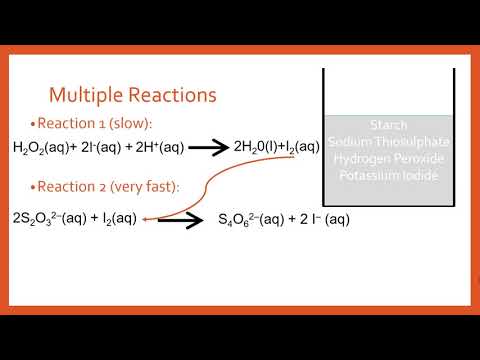
There are actually two chemical reactions going on at the same time when you combine the solutions. During these reactions two forms of iodine created – the elemental form and the ion form.
In Reaction # 1 iodide ions react with hydrogen peroxide to produce iodine element which is blue in the presence of starch. BUT, before that can actually happen, the Vitamin C quickly reacts and consumes the elemental iodine.
The net result, at least for part of the time is that the solution remains colorless with excess of iodide ions being present. Now after a short time as the reactions keep proceeding in this fashion, the Vitamin C gets gradually used up. Once the Vitamin C is used up, the solution turns blue, because now the iodine element and starch are present.
Safety Precautions:
Be careful when working with the iodine – it stains, and it stains really well. Be very careful not to spill any of the solution.
Waste Disposal:
Dispose all liquids down the drain with plenty of water.
Imagination Station, Toledo's hands-on science center, is a vital non-profit organization that is an integral part of Toledo's economic, educational and social landscape. Imagination Station provides a critical layer of science enrichment by serving as an educational partner for teachers, schools and parents. It's with a thoughtful blend of exhibits, experiences, education and excitement that Imagination Station is inspiring future generations of scientists in Northwest Ohio. With more than 250 hands-on exhibits and demonstrations, Imagination Station delivers a multi-sensory experience that's as fun as it is educational.
What you need:
distilled water (tap water will work OK as well)
a couple plastic cups
1000 mg vitamin C tablets
tincture of iodine (2%)
hydrogen peroxide (3%)
liquid laundry starch
What to do:
Make a vitamin C solution by crushing a 1000 mg vitamin C tablet and dissolving it in 2 oz of water. Label this as “vitamin C stock solution”.
Combine 1 tsp of the vitamin C stock solution with 1 tsp of iodine and 2 oz of water. Label this “solution A”.
Prepare “solution B” by adding 2 oz of water to 3 tsp of hydrogen peroxide and 1/2 tsp of liquid starch solution.
Pour solution A into solution B, and pour the resulting solution back into the empty cup to mix them thoroughly. Keep pouring the liquid back and fourth between the cups.
What’s going on?
There are actually two chemical reactions going on at the same time when you combine the solutions. During these reactions two forms of iodine created – the elemental form and the ion form.
In Reaction # 1 iodide ions react with hydrogen peroxide to produce iodine element which is blue in the presence of starch. BUT, before that can actually happen, the Vitamin C quickly reacts and consumes the elemental iodine.
The net result, at least for part of the time is that the solution remains colorless with excess of iodide ions being present. Now after a short time as the reactions keep proceeding in this fashion, the Vitamin C gets gradually used up. Once the Vitamin C is used up, the solution turns blue, because now the iodine element and starch are present.
Safety Precautions:
Be careful when working with the iodine – it stains, and it stains really well. Be very careful not to spill any of the solution.
Waste Disposal:
Dispose all liquids down the drain with plenty of water.
Imagination Station, Toledo's hands-on science center, is a vital non-profit organization that is an integral part of Toledo's economic, educational and social landscape. Imagination Station provides a critical layer of science enrichment by serving as an educational partner for teachers, schools and parents. It's with a thoughtful blend of exhibits, experiences, education and excitement that Imagination Station is inspiring future generations of scientists in Northwest Ohio. With more than 250 hands-on exhibits and demonstrations, Imagination Station delivers a multi-sensory experience that's as fun as it is educational.
Комментарии
 0:01:34
0:01:34
 0:00:15
0:00:15
 0:01:09
0:01:09
 0:00:42
0:00:42
 0:09:35
0:09:35
 0:00:12
0:00:12
 0:02:02
0:02:02
 0:03:36
0:03:36
 0:00:28
0:00:28
 0:00:17
0:00:17
 0:02:26
0:02:26
 0:05:46
0:05:46
 0:00:34
0:00:34
 0:01:28
0:01:28
 0:02:39
0:02:39
 0:12:25
0:12:25
 0:00:31
0:00:31
 0:06:24
0:06:24
 0:01:15
0:01:15
 0:02:54
0:02:54
 0:02:09
0:02:09
 0:05:26
0:05:26
 0:07:37
0:07:37
 0:05:08
0:05:08