filmov
tv
Iodine Clock Reaction Rates Of Reaction Lab

Показать описание
Iodine Clock Reaction Rates Of Reaction Lab
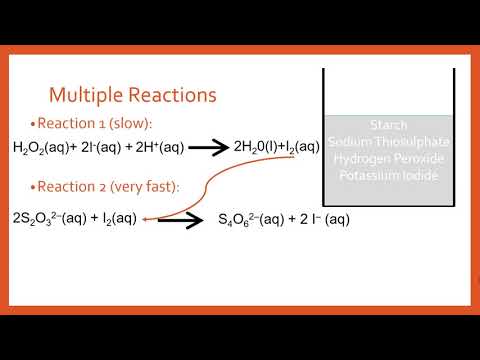
In this video I go through the effect of different concentrations on reaction rates for the Iodine clock reaction.
As the concentration of solution A decreases you should notice an indirect/inverse relationship between the time before the color change and endpoint of the reaction occurs with it becoming longer.
Be sure to take times for each trial by starting your stop watch when the first drops of liquid B make contact with liquid A, then clicking your stop watch to end the time when it changes the typical blue black color associated with an iodide starch test.
Keywords:
iodine clock reaction, iodine starch test, rates of reaction lab, high school chemistry lab, regents chemistry lab, stop reaction,
See all Chemistry reactions, calculations and tutorials here:
In this video I go through the effect of different concentrations on reaction rates for the Iodine clock reaction.
As the concentration of solution A decreases you should notice an indirect/inverse relationship between the time before the color change and endpoint of the reaction occurs with it becoming longer.
Be sure to take times for each trial by starting your stop watch when the first drops of liquid B make contact with liquid A, then clicking your stop watch to end the time when it changes the typical blue black color associated with an iodide starch test.
Keywords:
iodine clock reaction, iodine starch test, rates of reaction lab, high school chemistry lab, regents chemistry lab, stop reaction,
See all Chemistry reactions, calculations and tutorials here:
 0:03:36
0:03:36
 0:01:34
0:01:34
 0:01:09
0:01:09
 0:00:15
0:00:15
 0:07:37
0:07:37
 0:00:42
0:00:42
 0:04:43
0:04:43
 0:02:02
0:02:02
 0:05:21
0:05:21
 0:00:17
0:00:17
 0:03:49
0:03:49
 0:03:47
0:03:47
 0:06:01
0:06:01
 0:02:35
0:02:35
 0:07:05
0:07:05
 0:12:25
0:12:25
 0:00:24
0:00:24
 0:00:30
0:00:30
 0:17:52
0:17:52
 0:07:10
0:07:10
 0:15:26
0:15:26
 0:10:44
0:10:44
 0:15:01
0:15:01
 0:00:12
0:00:12