filmov
tv
SN2 Practice Questions

Показать описание
In this video I'm going to go over some of the most typical SN2-related questions you might see on the exam. I'm also including some tricky ones so you know what to be prepared for.
00:00 Intro and review of the SN2 reactions
01:48 Question 1 - predicting the products
06:02 Question 2 - tricky questions
11:07 Question 3 - rankings
👋 Connect with me on social media:
00:00 Intro and review of the SN2 reactions
01:48 Question 1 - predicting the products
06:02 Question 2 - tricky questions
11:07 Question 3 - rankings
👋 Connect with me on social media:
SN2 Practice Questions
SN2 Reaction Practice Problems
Choosing Between SN2, SN1, E2 and E1 Reactions
Practice drawing SN1 vs SN2 reaction mechanisms and products with more than 9 examples
Practice Problem: Drawing Substitution and Elimination Products (SN1/SN2/E1/E2)
Substitution and Elimination Practice Questions | SN1 SN2 E1 E2
SN2 SN1 E1 E2 Reaction Mechanisms Made Easy!
Organic Chemistry: SN2 Practice Problems! (Exam Questions!) (Substitution Reactions)
Substitution and Elimination SN1 SN2 E1 E2 Practice Questions: Part 2
SN2 Reaction Mechanisms
SN1/SN2/E1/E2 - working through problems!
SN1 SN2 E1 E2 Reaction Mechanism - Test Review
Choosing Between SN1/SN2/E1/E2 Mechanisms
advanced SN2 reaction practice
Choosing substrates for an SN2 reaction #organicchemistry
Question 1: Is this an SN1 or SN2 reaction? #organicchemistry
SN1 SN2 E1 E2 Pre-Finals Practice [Live Recording] Organic Chemistry Review
Organic Chemistry | E1, E2, SN1 and SN2 Practice Question - Rearrangement.
Predicting Products of SN2 Reactions
SN1 SN2 E1 E2 Pre-Finals Practice (Live Recording) Organic Chemistry Review
Choosing Between SN1 SN2 E1 E2 Reactions
SN1 SN2 E1 E2 Reaction Mechanism Overview
classifying SN2, SN1, E2, & E1 reactions
Determining SN1, SN2, E1, and E2 Reactions: Crash Course Organic Chemistry #23
Комментарии
 0:16:36
0:16:36
 0:14:32
0:14:32
 0:09:06
0:09:06
 0:23:01
0:23:01
 0:06:52
0:06:52
 0:14:51
0:14:51
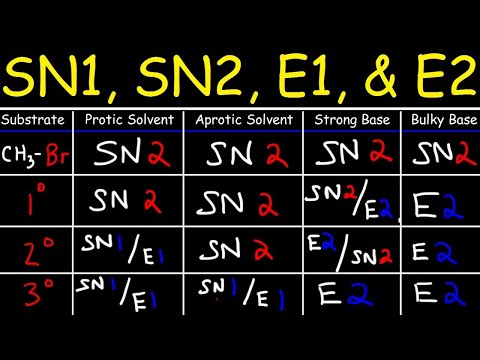 0:38:50
0:38:50
 0:18:39
0:18:39
 0:33:26
0:33:26
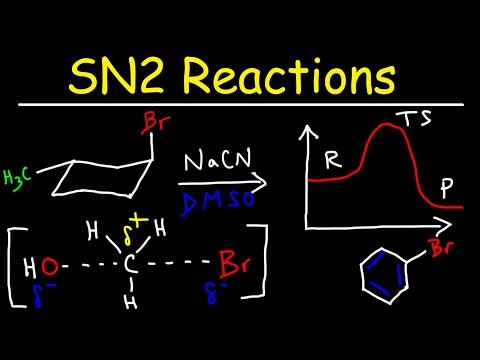 0:22:49
0:22:49
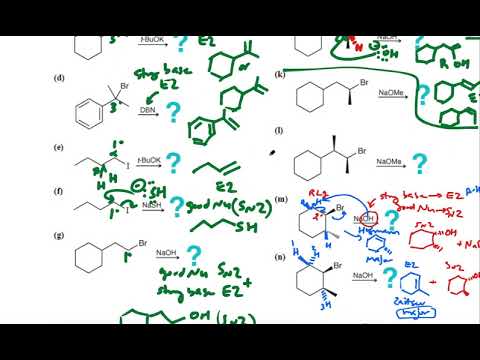 0:14:34
0:14:34
 0:59:10
0:59:10
 0:18:52
0:18:52
 0:03:06
0:03:06
 0:00:31
0:00:31
 0:00:59
0:00:59
 0:56:34
0:56:34
 0:10:54
0:10:54
 0:05:35
0:05:35
 0:58:27
0:58:27
 0:12:31
0:12:31
 0:07:29
0:07:29
 0:19:08
0:19:08
 0:13:31
0:13:31