filmov
tv
Viewer's Request: Algebra #2: Mixture Problem

Показать описание
To donate:
We will solve:
If a 30% solution is mixed with a 60% solution and 10kg of 0% solution, you obtain a 36%. But if they are mixed with 10kg of 50% solution you obtain a 41% solution. How much of each solution are you mixing?
Next video in this series can be seen at:
Viewer's Request: Algebra #2: Mixture Problem
Viewer's Request: Algebra #1: Distance, Rate, and Time
mixture problem three variables
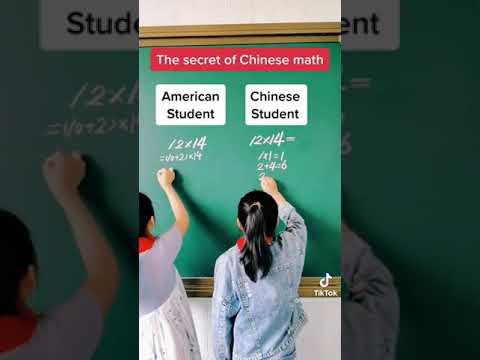
HOW CHINESE STUDENTS SO FAST IN SOLVING MATH OVER AMERICAN STUDENTS
Salsa Night in IIT Bombay #shorts #salsa #dance #iit #iitbombay #motivation #trending #viral #jee
Distance, Rate, Time Problems - Algebra 2 for Teens!
Logical Reasoning???#viral #vidumzn
Physics: Viewer's Request: Mechanics #24: Impulse on a Revolving Object
Algebra - Ch. 30: Linear Equations (4 of 33) How to Plot Points?
Physics: Viewer's Request: Mechanics #8: Kinematics - What is the Average Speed? Method 2
Steam at 2 MPa and 250°C in a rigid cylinder is cooled until the quality is 30%. Find the heat re......
Physics: Viewer's Request: Mechanics #12: Projectile Motion (with Lots of Unknowns)
Multidiscipline Design Optimization
Determine the air-standard efficiency of an engine operating on the diesel cycle with a clearance...
RATIO FORMULA, ANALYSIS ,INTERPRETATION,GRAPH AND TABLE WITH SIMPLE EXAMPLE TO UNDERSTAND
Physics: Viewer's Request: Mechanics #31: Torque=? Momentum=? Impulse=? Acceleration=? Inertia=...
Pre-U Physics: Kinematics Booklet 4: Section B
L#4 | 2022 | Computer Algebra System and Related Softwares | WORKING WITH HISTORY COMMANDS IN R | DU
Problem-Solving with Units
A perfect gas has a value of R = 58.8 ft-lb/lb.-°R and k = 1.26. If 20 Btu are added to 10 lbs...
A refrigeration plant is rated at 15 tons capacity. How many pounds of air per hour will it cool...
Inequalities Part 2 (Reasoning)
Compute the mass of a 2 m3 propane at 280 kPa and 40°C
Mixture and Alligation Most Important Practice Questions and Solutions For Government Job Exams.
Комментарии
 0:09:13
0:09:13
 0:10:55
0:10:55
 0:09:10
0:09:10
 0:00:23
0:00:23
 0:00:14
0:00:14
 0:12:37
0:12:37
 0:00:11
0:00:11
 0:04:30
0:04:30
 0:03:49
0:03:49
 0:09:03
0:09:03
 0:04:16
0:04:16
 0:05:24
0:05:24
 0:13:13
0:13:13
 0:06:05
0:06:05
 0:09:40
0:09:40
 0:09:56
0:09:56
 0:15:07
0:15:07
 0:42:00
0:42:00
 0:24:02
0:24:02
 0:05:02
0:05:02
 0:04:04
0:04:04
 0:09:45
0:09:45
 0:02:39
0:02:39
 0:23:47
0:23:47