filmov
tv
The Law of Conservation of Mass - MeitY OLabs

Показать описание
This video channel is developed by Amrita University's CREATE
▶ For more Information @
▶ Amrita Online Lab Project Website
▶ Subscribe @
▶ Like us @
Copyright © 2017 Amrita University
Developed by Amrita University & CDAC Mumbai. Funded by MeitY (Ministry of Electronics & Information Technology)
The Law of Conservation of Mass:-
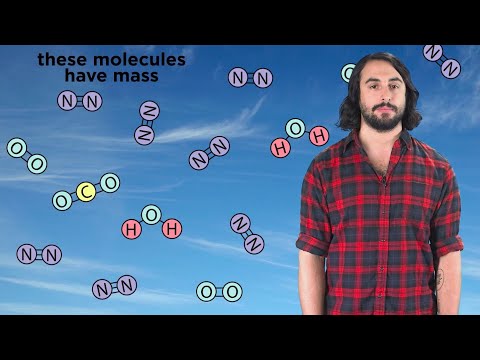
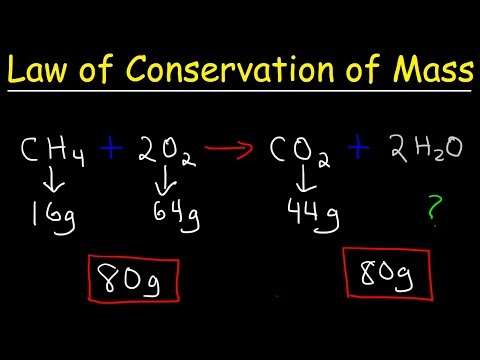
A French chemist, Antoine Lavoisier, who is known as the father of modern chemistry, changed chemistry from a qualitative to a quantitative science. He proved that the mass of the products in a chemical reaction is equal to the mass of the reactants. There are no more atoms at the end of the chemical reaction than there were at the beginning.
" The Law of Conservation of Mass states that matter can neither be created nor destroyed in a chemical reaction" . It is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
This video explains how to verify the Law of Conservation of Mass during a chemical reaction.
▶ For more Information @
▶ Amrita Online Lab Project Website
▶ Subscribe @
▶ Like us @
Copyright © 2017 Amrita University
Developed by Amrita University & CDAC Mumbai. Funded by MeitY (Ministry of Electronics & Information Technology)
The Law of Conservation of Mass:-
A French chemist, Antoine Lavoisier, who is known as the father of modern chemistry, changed chemistry from a qualitative to a quantitative science. He proved that the mass of the products in a chemical reaction is equal to the mass of the reactants. There are no more atoms at the end of the chemical reaction than there were at the beginning.
" The Law of Conservation of Mass states that matter can neither be created nor destroyed in a chemical reaction" . It is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
This video explains how to verify the Law of Conservation of Mass during a chemical reaction.
 0:04:37
0:04:37
 0:05:53
0:05:53
 0:03:48
0:03:48
 0:10:06
0:10:06
 0:01:58
0:01:58
 0:03:14
0:03:14
 0:01:33
0:01:33
 0:03:05
0:03:05
 0:07:56
0:07:56
 0:01:39
0:01:39
 0:10:10
0:10:10
 0:03:56
0:03:56
 0:02:39
0:02:39
 0:05:53
0:05:53
 0:05:25
0:05:25
 0:02:33
0:02:33
 0:15:11
0:15:11
 0:10:59
0:10:59
 0:02:46
0:02:46
 0:17:01
0:17:01
 0:02:37
0:02:37
 0:03:58
0:03:58
 0:00:25
0:00:25
 0:03:51
0:03:51