filmov
tv
Law of Conservation of Mass

Показать описание
This lecture is about law of conservation of mass. . Also, you will learn that how mass of the reactants is equal to the mass of product in any chemical reaction.
Q: What is the law of conservation of mass?.
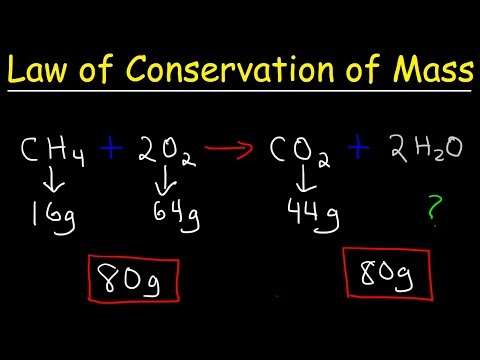
Ans: The easy definition of law of conservation of mass is “Mass or matter can neither be created nor destroyed and chemical reaction, the total mass of the reactants is equal to the mass of products”.
For example, when 2 grams of hydrogen react with 16 grams of oxygen, they form 18 grams of water. In this chemical reaction, no mass is destroyed and no mass is created.
Therefore, we say that the mass of the reactants and products remains served in a chemical reaction. This is also called conservation of mass or conservation of matter.
To learn more about the law of conservation of mass, watch this animated lecture till the end.
#LawOfConservationOfMass
#LawOfConservationOfMatter
#Chemistry
Q: What is the law of conservation of mass?.
Ans: The easy definition of law of conservation of mass is “Mass or matter can neither be created nor destroyed and chemical reaction, the total mass of the reactants is equal to the mass of products”.
For example, when 2 grams of hydrogen react with 16 grams of oxygen, they form 18 grams of water. In this chemical reaction, no mass is destroyed and no mass is created.
Therefore, we say that the mass of the reactants and products remains served in a chemical reaction. This is also called conservation of mass or conservation of matter.
To learn more about the law of conservation of mass, watch this animated lecture till the end.
#LawOfConservationOfMass
#LawOfConservationOfMatter
#Chemistry
Law of Conservation of Mass - Fundamental Chemical Laws, Chemistry
The law of conservation of mass - Todd Ramsey
GCSE Chemistry - Conservation of Mass #26
What Is The Law of Conservation of Mass | Properties of Matter | Chemistry | FuseSchool
Law of Conservation of Mass | Don't Memorise
The Law of Conservation of Matter
Law of Conservation of Mass Example
Law of Conservation of Mass
Balance Chemical Equation | class 10 Chapter - 1
Law of conservation of mass | Atoms and Molecules | Chemistry | Khan Academy
Law of Conservation of Mass experiment | Law of conservation of matter | Chemistry
Law of conservation of mass: demonstration
The Law of Conservation of Mass - MeitY OLabs
The Law of Conservation of Matter
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3
Law of Conservation of Mass
Conservation Of Mass Grade 10
Vinegar + Baking Soda - Law of Conservation of Mass
Law of Conservation of Mass Word Problems
Law of Conservation of Mass
Best Conservation of Mass Experiment EVER!
The Law of Conservation of Mass - OLabs
law of conservation of mass | arvind arora | a2motivation
Conservation of Mass
Комментарии
 0:03:14
0:03:14
 0:04:37
0:04:37
 0:04:04
0:04:04
 0:03:48
0:03:48
 0:03:05
0:03:05
 0:05:53
0:05:53
 0:01:39
0:01:39
 0:03:30
0:03:30
 0:23:40
0:23:40
 0:03:00
0:03:00
 0:01:25
0:01:25
 0:02:18
0:02:18
 0:02:33
0:02:33
 0:01:58
0:01:58
 0:10:59
0:10:59
 0:04:11
0:04:11
 0:10:41
0:10:41
 0:02:39
0:02:39
 0:02:07
0:02:07
 0:06:21
0:06:21
 0:01:00
0:01:00
 0:03:51
0:03:51
 0:03:44
0:03:44
 0:02:27
0:02:27