filmov
tv
Calculating the Concentration of a Complex Ion 001

Показать описание
You convert Zn(H2O)42+ to the more stable Zn(NH3)42+ by mixing 50.0 L of 0.0020 M Zn(H2O)42+ and 25.0 L of 0.15 M NH3. What is the final [Zn(H2O)42+]? Kf of Zn(NH3)42+ = 7.8 x 108.
Concentration Formula & Calculations | Chemical Calculations | Chemistry | Fuse School
GCSE Chemistry - How to Calculate Concentration in grams per decimetre cubed #30
Concentration grade 10
GCSE Chemistry - Moles, Concentration & Volume Calculations #29
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
Concentration | How to Calculate Concentration of a Solution?
Concentration of solutions Chemistry
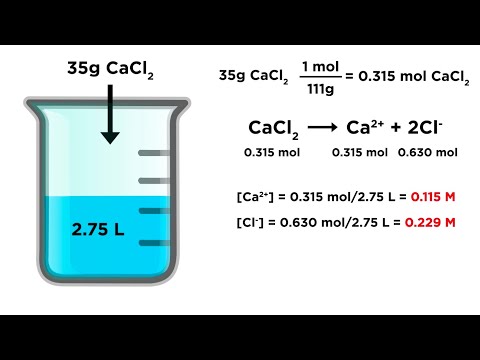
Calculating Ion Concentrations in Solution
GRADE 12 ACIDS AND BASES (PART5) CONCENTRATION CALCULATIONS
Ion Concentration in Solutions From Molarity, Chemistry Practice Problems
How to solve percent concentration problems even if you're 🤷🏻♀️ - Dr K
Concentration and Molarity explained: what is it, how is it used + practice problems
Calculating the Concentration in g/dm3
Calculating the Concentration of a Standardized Solution
Calculating the concentration of a solution using a dilution factor
Worked example: Calculating concentration using the Beer–Lambert law | AP Chemistry | Khan Academy
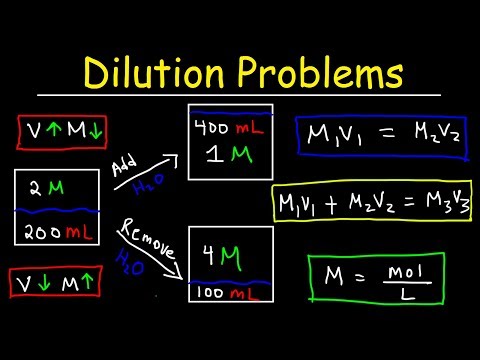
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
How to calculate concentration from pH and pOH
How to calculate percent concentration | Percent mass | Percent volume | Percent mass-volume - Dr K
ALEKS: Calculating mass concentration
Concentration and Dilution of Solutions
Calculating the Concentration of a Complex Ion 001
DON'T STRUGGLE with Concentration calculations in mol per dm3. Watch this video!
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Комментарии
 0:04:25
0:04:25
 0:03:28
0:03:28
 0:23:41
0:23:41
 0:06:04
0:06:04
 0:31:25
0:31:25
 0:05:29
0:05:29
 0:09:27
0:09:27
 0:05:05
0:05:05
 0:08:19
0:08:19
 0:12:24
0:12:24
 0:05:51
0:05:51
 0:05:41
0:05:41
 0:04:04
0:04:04
 0:07:55
0:07:55
 0:04:40
0:04:40
 0:03:48
0:03:48
 0:21:55
0:21:55
 0:03:14
0:03:14
 0:03:33
0:03:33
 0:03:24
0:03:24
 0:06:17
0:06:17
 0:11:41
0:11:41
 0:13:09
0:13:09
 0:08:46
0:08:46