filmov
tv
Calculating Ion Concentrations in Solution
Показать описание
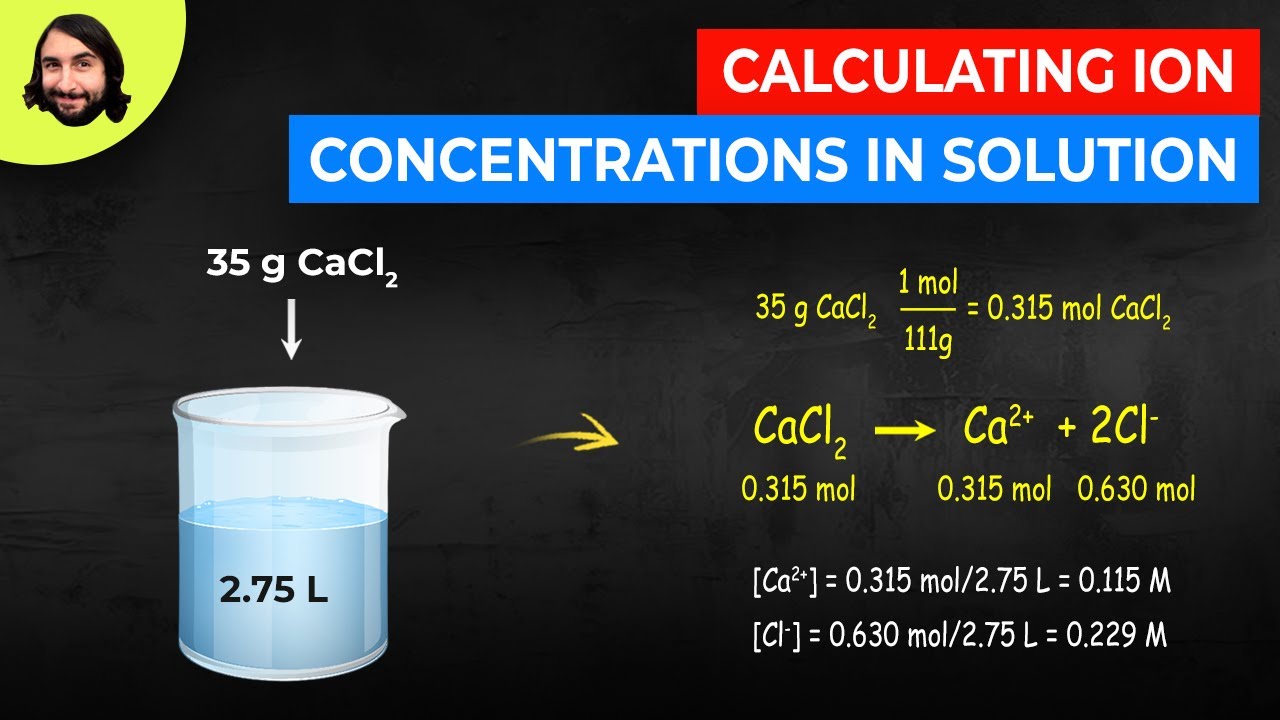
We know that concentration is typically expressed with molarity, which is moles per liter. But how do we know how many moles of solute are present in solution when an ionic solid dissolves in water? It's not so simple because these will dissociate into individual ions, so there is some extra stoichiometry to do!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Calculating Ion Concentrations in Solution
Ion Concentration in Solutions From Molarity, Chemistry Practice Problems
Concentration of ions when mixing solutions
Molarity of Ions - Calculating Concentration of Ions in a Solution - Straight Science
Ions in Solution After Precipitation
Hydrogen Ion and Hydroxide Ion Concentrations (Example)
Finding the concentration of ions in an aqueous solution
Ion Concentration
Chemistry Nov 2005: Grade 12/ A final revision walk through @ zamokuhlemakhamba
4.2 Concentration of Ions in Solution
Ksp: Concentration of Ions in Solution
CHEMISTRY 101: Calculating Ion Concentration When Adding Together Two Solutions
How to Calculate Hydrogen Ion Concentration from pH
How To Calculate The Hydroxide Ion Concentration | Chemistry
03 Calculating Ion Concentrations
CHEM 101 - Calculating Ion Concentration When Adding Together Two Solutions Part 2
How to Calculate the Molarity of Ions in a Solution?
How to solve ion concentrations in solutions
Determining Concentration of Ions in Voltaic Cell
Chemistry Problem Solving: How to calculate ion concentration in an aqueous solution
CHEMISTRY 101: Calculating Ion Concentration by Molarity and Solution Dilution
How to Calculate the Hydrogen Ion Concentration of a Weak Acid Solution
Concentration of different ions in a solution
Calculating Hydrogen Ion Concentration
Комментарии
 0:05:05
0:05:05
 0:12:24
0:12:24
 0:14:44
0:14:44
 0:05:08
0:05:08
 0:09:33
0:09:33
 0:02:09
0:02:09
 0:01:48
0:01:48
 0:04:50
0:04:50
 5:07:33
5:07:33
 0:02:54
0:02:54
 0:03:29
0:03:29
 0:02:31
0:02:31
 0:01:57
0:01:57
 0:12:32
0:12:32
 0:06:17
0:06:17
 0:02:50
0:02:50
 0:05:05
0:05:05
 0:12:27
0:12:27
 0:12:50
0:12:50
 0:02:02
0:02:02
 0:03:17
0:03:17
 0:09:28
0:09:28
 0:04:21
0:04:21
 0:10:14
0:10:14