filmov
tv
How to Name Ionic Compounds with Polyatomic Ions
Показать описание
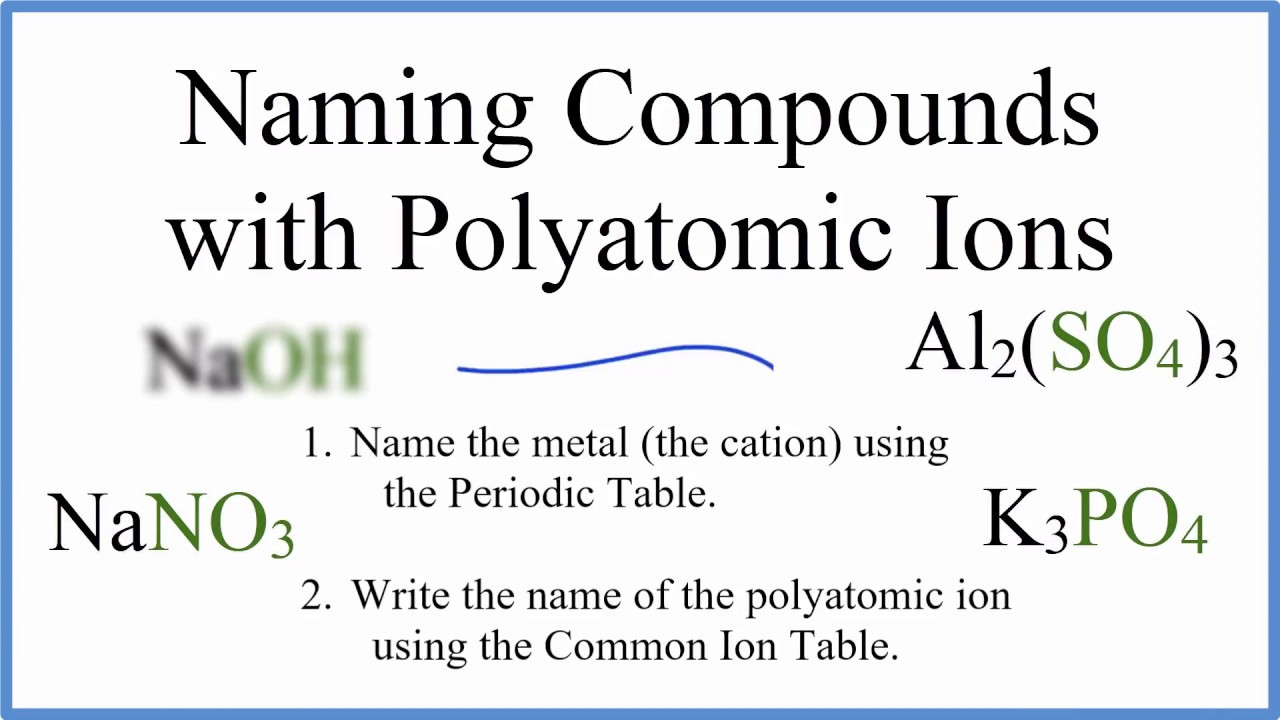
In this video we'll write the correct name for ionic compounds with polyatomic ions (ionic compounds with three different elements).
These compounds have polyatomic ions and we’ll use the Common Ion Table (a list of polyatomic ions) to help us write the name when given the formula.
To write the name for ionic compounds with polyatomic ions we’ll use the Periodic Table and the Common Ion Table and follow some simple rules.
---Keys for Naming Ternary Ionic Compounds---
- Name the metal (the cation) as it appears on the Periodic Table.
Na+ = Sodium Al3+ = Aluminum
- Find the polyatomic ion on the Common Ion Table and write the name.
Note: It is possible to have two polyatomic ions such as NH4NO3. In this case find and write both names as found on the Common Ion Table.
For a complete tutorial on naming and formula writing for compounds visit:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
These compounds have polyatomic ions and we’ll use the Common Ion Table (a list of polyatomic ions) to help us write the name when given the formula.
To write the name for ionic compounds with polyatomic ions we’ll use the Periodic Table and the Common Ion Table and follow some simple rules.
---Keys for Naming Ternary Ionic Compounds---
- Name the metal (the cation) as it appears on the Periodic Table.
Na+ = Sodium Al3+ = Aluminum
- Find the polyatomic ion on the Common Ion Table and write the name.
Note: It is possible to have two polyatomic ions such as NH4NO3. In this case find and write both names as found on the Common Ion Table.
For a complete tutorial on naming and formula writing for compounds visit:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Комментарии
 0:13:33
0:13:33
 0:05:44
0:05:44
 0:11:25
0:11:25
 0:10:32
0:10:32
 0:10:10
0:10:10
 0:00:53
0:00:53
 0:03:57
0:03:57
 0:10:22
0:10:22
 1:21:37
1:21:37
 0:03:23
0:03:23
 0:07:14
0:07:14
 0:06:39
0:06:39
 0:06:07
0:06:07
 0:02:15
0:02:15
 0:15:31
0:15:31
 0:01:30
0:01:30
 0:02:22
0:02:22
 0:11:44
0:11:44
 0:10:44
0:10:44
 0:06:05
0:06:05
 0:11:58
0:11:58
 0:06:08
0:06:08
 0:04:08
0:04:08
 0:05:22
0:05:22