filmov
tv
OCR A 3.2.1 Enthalpy changes REVISION

Показать описание
OCR A 3.2.1 Enthalpy changes REVISION
The EASIEST Method For Solving Hess Cycles
Enthalpy Changes A* Revision OCR A-Level Chemistry
OCR AS Chemistry Unit 2 Module 3 - Determining enthalpy changes from surroundings
Required practical 2: Measurement of an enthalpy change
Calculating enthalpy change
Exam Walkthrough - Enthalpy Changes
Introduction to Enthalpy | A-level Chemistry | OCR, AQA, Edexcel
OCR Chemistry Unit 2 - Enthalpy changes
Quick Revision - Enthalpy changes from equations
Hess' Law and Enthalpy Cycles | A-level Chemistry | OCR, AQA, Edexcel
Hess’ Law Cycle (using enthalpy changes of formation)
Measuring Enthalpy Change Part 1 Enthalpy of Fuels
A Level Chemistry - 11a - Enthalpy Changes
PRACTICAL SKILLS - PAG 3 - ENTHALPY DETERMINATION
Enthalpy Change (AQA A level Chemistry)
OCR chemistry MORE ENTHALPY CHANGES
How an A* student answers Enthalpy Changes Multiple Choice Question OCR #alevelchemistry
Calculating Enthalpy Change of Solution | A-Level Chemistry | AQA, OCR, Edexcel
Hess's Law
Past Question Walkthrough - Enthalpy Changes 1
OCR A level Chemistry Unit F322 Module 3 - Bond enthalpy
Calculating a bond enthalpy from the enthalpy change
Determining Enthalpy Change Experimentally AND Hess Cycles (A Level Chemistry)
Комментарии
 0:23:26
0:23:26
 0:13:46
0:13:46
 0:09:19
0:09:19
 0:15:15
0:15:15
 0:07:09
0:07:09
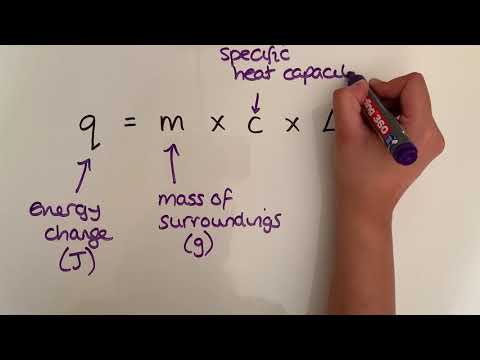 0:07:22
0:07:22
 0:07:28
0:07:28
 0:14:16
0:14:16
 0:11:11
0:11:11
 0:06:29
0:06:29
 0:24:15
0:24:15
 0:05:24
0:05:24
 0:10:38
0:10:38
 0:15:16
0:15:16
 0:07:12
0:07:12
 0:22:25
0:22:25
 0:16:57
0:16:57
 0:00:38
0:00:38
 0:03:20
0:03:20
 0:17:55
0:17:55
 0:06:14
0:06:14
 0:07:47
0:07:47
 0:03:09
0:03:09
 0:11:35
0:11:35