filmov
tv
Calculate the standard free enegry change for the reaction: `Zn + Cu^(2+)(aq) rarr Cu+Zn^(2+) (aq)

Показать описание
Calculate the standard free enegry change for the reaction: `Zn + Cu^(2+)(aq) rarr Cu+Zn^(2+) (aq), E^(Theta) = 1.20V`
 0:02:02
0:02:02
 0:01:53
0:01:53
 0:04:05
0:04:05
 0:04:28
0:04:28
 0:10:53
0:10:53
 0:06:05
0:06:05
 0:02:03
0:02:03
 0:11:32
0:11:32
 1:01:04
1:01:04
 0:06:04
0:06:04
 0:02:03
0:02:03
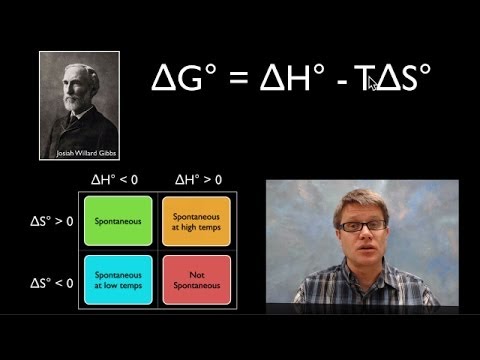 0:07:57
0:07:57
 0:15:05
0:15:05
 0:03:29
0:03:29
 0:07:10
0:07:10
 0:07:05
0:07:05
 0:03:26
0:03:26
 0:04:57
0:04:57
 0:04:59
0:04:59
 0:04:06
0:04:06
 0:08:12
0:08:12
 0:12:38
0:12:38
 0:04:06
0:04:06
 0:05:47
0:05:47