filmov
tv
Chemistry Lesson: Acid-Base Neutralization Reactions
Показать описание
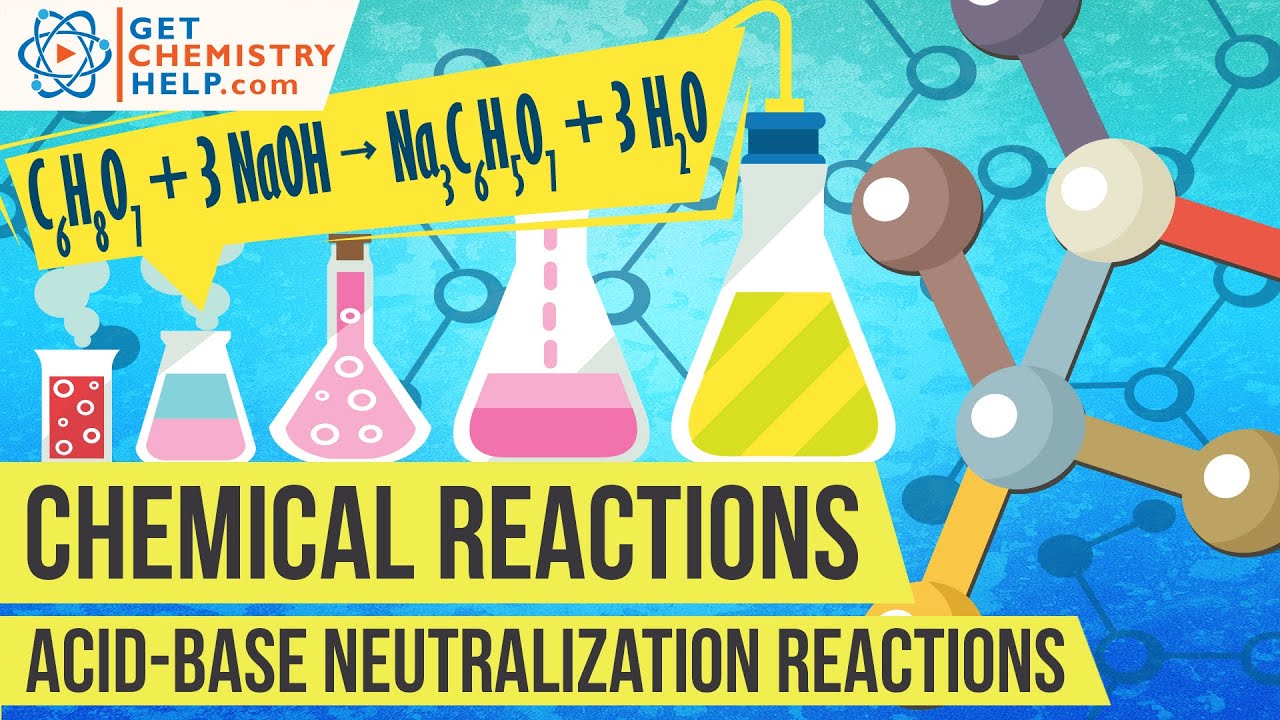
Neutralization reactions, or acid-base reactions, are a specialized type of double displacement reaction where an acid and base combine to form a salt (ionic compound) and water. In this lesson, we'll learn how to predict the products of neutralization reactions. I also include tips on balancing acid-base reactions.
WATCH FIRST:
WATCH NEXT:
LEARN CHEMISTRY FAST & EASY:
COME SAY HI!
In this chemistry lesson I cover several examples of acid base neutralization reactions and salts formed. Predicting products of neutralization reactions involves using a double displacement reaction where the products are always an ionic compound and water. The first example neutralization reaction is phosphoric acid and potassium hydroxide. The second acid-base reaction example is hydrobromic acid and calcium hydroxide. The third example of neutralization chemistry is sulfurous acid and lithium hydroxide (base). Enjoy this crash course on neutralization reactions of acids and bases!
WATCH FIRST:
WATCH NEXT:
LEARN CHEMISTRY FAST & EASY:
COME SAY HI!
In this chemistry lesson I cover several examples of acid base neutralization reactions and salts formed. Predicting products of neutralization reactions involves using a double displacement reaction where the products are always an ionic compound and water. The first example neutralization reaction is phosphoric acid and potassium hydroxide. The second acid-base reaction example is hydrobromic acid and calcium hydroxide. The third example of neutralization chemistry is sulfurous acid and lithium hydroxide (base). Enjoy this crash course on neutralization reactions of acids and bases!
Комментарии
 0:08:21
0:08:21
 0:13:33
0:13:33
 0:06:25
0:06:25
 0:04:35
0:04:35
 0:08:19
0:08:19
 0:08:21
0:08:21
 0:03:22
0:03:22
 0:11:17
0:11:17
 1:05:45
1:05:45
 0:06:50
0:06:50
 0:14:05
0:14:05
 0:10:51
0:10:51
 0:00:15
0:00:15
 0:05:13
0:05:13
 0:06:17
0:06:17
 0:10:22
0:10:22
 0:02:53
0:02:53
 0:00:54
0:00:54
 0:02:42
0:02:42
 0:02:16
0:02:16
 0:03:15
0:03:15
 0:04:37
0:04:37
 0:17:32
0:17:32
 0:00:15
0:00:15