filmov
tv
ABG Interpretation: The Anion Gap (Lesson 5)
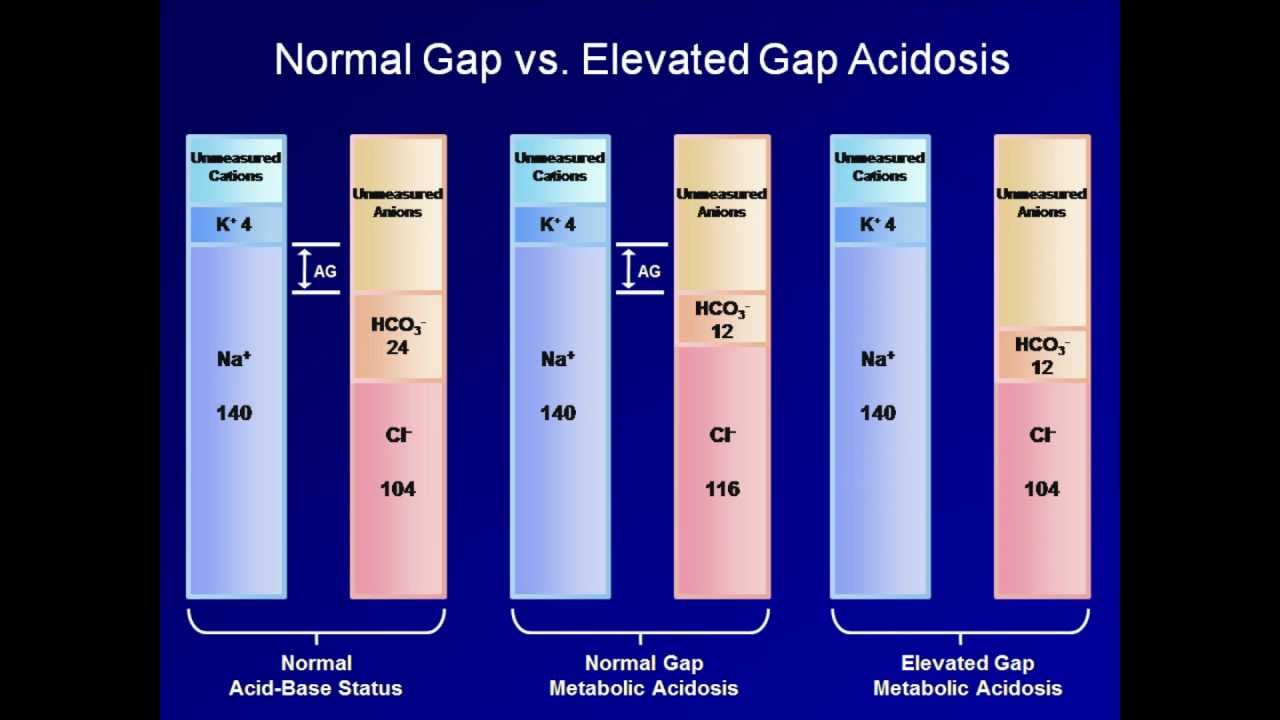
Показать описание
A discussion of the anion gap, including its calculation and its use in categorizing metabolic acidoses, including multiple examples of acid-base interpretation.
ABG Interpretation: The Anion Gap (Lesson 5)
Acid Base Disorders and ABG Interpretation | Introduction
Plasma anion gap
ANION GAP | Ridiculously simple
Elevated Anion Gap Metabolic Acidosis (ABG Interpretation - Lesson 8)
Anion Gap (AG) Calculation & Interpretation - Simply Explained
Normal Anion Gap Metabolic Acidosis (ABG Interpretation - Lesson 9)
ABG Interpretation: The Delta Ratio and Triple Disorders (Lesson 6)
Anion Gap Metabolic acidosis made easy ALL YOU NEED TO KNOW
Anion Gap EXPLAINED
How to interpret Arterial Blood Gas (ABG) reports : Master in 10 Mins: One shot
ABG Interpretation: A Grand Overview of Acid-Base Analysis (Lesson 13)
ABG Interpretation: Mixed Acid-Base Disorders with Normal pH (Lesson 7)
ABG Interpretation | Understanding Arterial Blood Gas Analysis - OSCE Guide | UKMLA | CPSA
Respiratory Therapy - Anion Gap - When to give bicarb?
Arterial blood gases interpretation anion gap 15/29(English)
Acidosis and Alkalosis MADE EASY
Respiratory Therapy - Advanced ABG Interpretations
Medical Acid Base Balance, Disorders & ABGs Explained Clearly (Remastered)
Delta Ratio (ABG Part - 3) | Concept and Clinical Significance.
ABG Interpretation - Metabolic Acidosis
ABG Interpretation: Compensation and Mixed Disorders (Lesson 4)
Interpret an Arterial Blood gas report in 4 steps
Arterial blood gases interpretation Urinary anion gap. 21/29(English)
Комментарии
 0:18:57
0:18:57
 0:58:08
0:58:08
 0:10:01
0:10:01
 0:08:32
0:08:32
 0:42:45
0:42:45
 0:10:00
0:10:00
 0:21:34
0:21:34
 0:21:14
0:21:14
 0:12:12
0:12:12
 0:06:54
0:06:54
 0:10:53
0:10:53
 0:33:26
0:33:26
 0:19:17
0:19:17
 0:10:29
0:10:29
 0:10:02
0:10:02
 0:22:06
0:22:06
 0:05:28
0:05:28
 0:44:24
0:44:24
 0:12:34
0:12:34
 0:09:27
0:09:27
 0:08:13
0:08:13
 0:17:12
0:17:12
 0:05:02
0:05:02
 0:10:03
0:10:03