filmov
tv
Giant Covalent Structures: Diamond, graphite and silicon dioxide. GCSE chemistry

Показать описание
How to relate the properties of diamond, graphite and silicon dioxide to their structures and bonding. Essential viewing for anyone studying GCSE Chemistry or GCSE Combined Science (trilogy). These are all examples of giant covalent molecules
If you would like to book 1 to 1 tuition with Mr B via Zoom, please use the following link to find out more:
If you would like to book 1 to 1 tuition with Mr B via Zoom, please use the following link to find out more:
GCSE Chemistry - Allotropes of Carbon - Diamond and Graphite #18
GCSE Chemistry - Properties of Simple Molecular Substances & Giant Covalent Structures #17
Giant Covalent Structures. Diamond. Graphite and Quartz.
What Are Giant Chemical Structures | Properties of Matter | Chemistry | FuseSchool
Structure of Diamond and Graphite, Properties - Basic Introduction
Giant Covalent Structures like diamond and graphite for GCSE Chemistry
Giant Covalent Structures: Diamond, graphite and silicon dioxide. GCSE chemistry
3.6.1 Describe the giant covalent structures of graphite and diamond.
GCSE science and chemistry (9-1)-The structures and properties of diamond, graphite and silica
Molecular Structures Of Diamond & Graphite, Easy & Budget Friendly School Project.
Giant Covalent Structures
GCSE C3 Giant covalent structures
IGCSE/O Level Lecture #6 Giant Covalent Structures of Diamond, Graphite and Silica.
Giant Covalent Structures(Diamond,graphite and silicon(IV)oxide)
Giant Covalent Structures - AS Chemistry
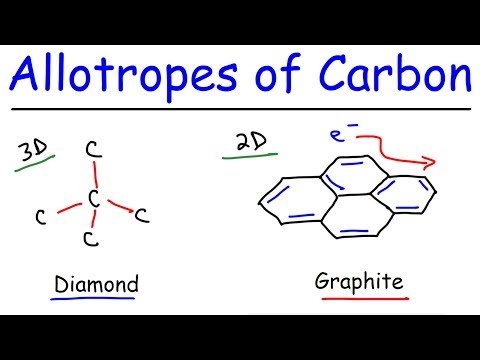
Allotropes of Carbon - Graphite, Diamond, Graphene, & Fullerenes
Giant covalent structures and their properties for GCSE science
Giant covalent structures
Giant covalent structures - diamond and graphite
GIANT COVALENT STRUCTURES - Chemistry Science Revision (GCSE) #school #exams #diamond #graphite
how to draw diamond structure #shorts #youtubeshorts #reels #chemistry #shortvideo #viralvideo
Giant covalent structures:Diamond and graphite
How to draw a diamond tetrahedral giant covalent structure
What Are Allotropes? Non-Metals | Properties of Matter | Chemistry | FuseSchool
Комментарии
 0:03:22
0:03:22
 0:04:46
0:04:46
 0:01:40
0:01:40
 0:02:30
0:02:30
 0:14:28
0:14:28
 0:06:53
0:06:53
 0:09:04
0:09:04
 0:02:16
0:02:16
 0:07:11
0:07:11
 0:03:59
0:03:59
 0:06:05
0:06:05
 0:01:11
0:01:11
 0:48:56
0:48:56
 0:14:34
0:14:34
 0:20:25
0:20:25
 0:06:33
0:06:33
 0:03:43
0:03:43
 0:09:20
0:09:20
 0:04:24
0:04:24
 0:00:59
0:00:59
 0:01:00
0:01:00
 0:06:04
0:06:04
 0:02:21
0:02:21
 0:04:37
0:04:37