filmov
tv
Structure of Diamond and Graphite, Properties - Basic Introduction
Показать описание
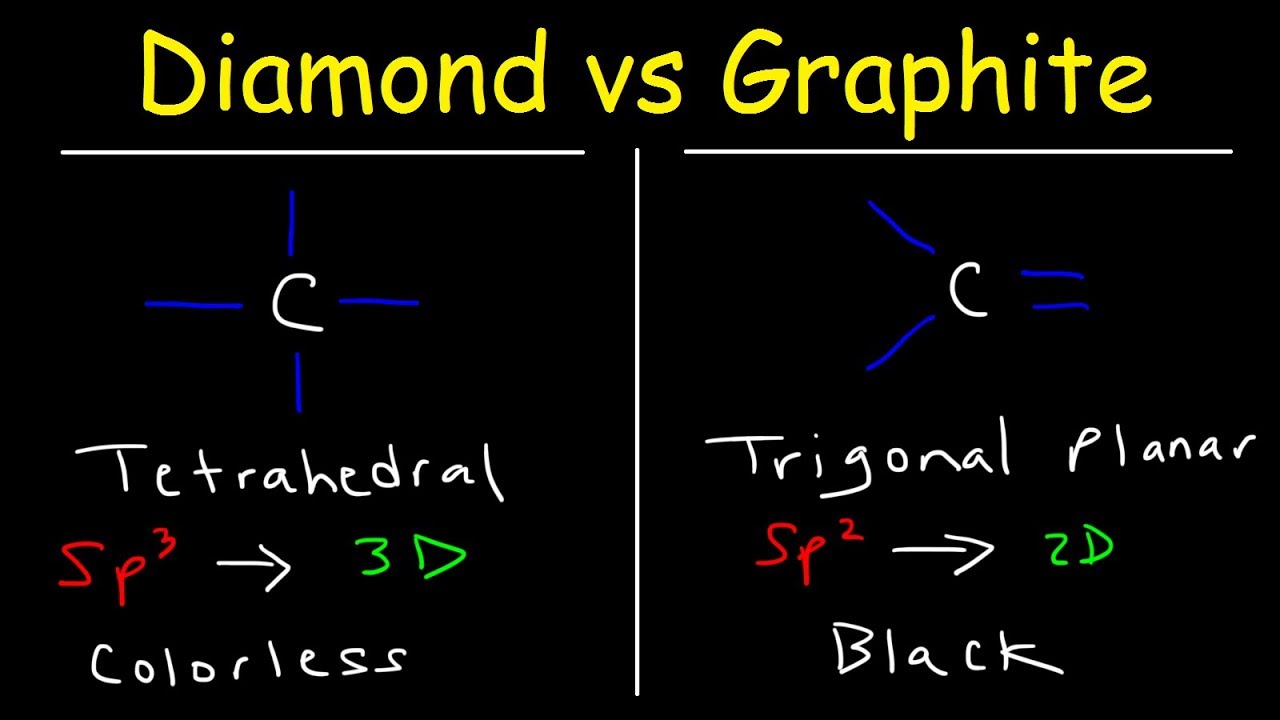
This chemistry video tutorial provides a basic introduction into the structure of diamond and graphite. Diamond has a tetrahedral geometry around each carbon atom with an sp3 hybridization. Graphite has a trigonal planar geometry around each carbon atom with an sp2 hybridization. Graphite conducts electricity due to the electron delocalization of its pi electrons where as diamond as an electrical insulator due to its localized valence electrons; however, diamond is excellent conductor of heat. Diamond is colorless and graphite is black. Diamond is hard due to its strong network covalent bonds where as graphite is slippery due to the weak interactions between the 2D layers within the structure of graphite.
Sigma and Pi Bonding:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
_______________________________
Hydrogen Bonding:
London Dispersion Forces:
Ion Dipole Forces:
Bragg's Equation For X-Ray Diffraction:
Molecular & Network Covalent Solids:
_______________________________
Metallic Bonding:
Metal Alloys:
Diamond Vs Graphite:
Semiconductors:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Sigma and Pi Bonding:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
_______________________________
Hydrogen Bonding:
London Dispersion Forces:
Ion Dipole Forces:
Bragg's Equation For X-Ray Diffraction:
Molecular & Network Covalent Solids:
_______________________________
Metallic Bonding:
Metal Alloys:
Diamond Vs Graphite:
Semiconductors:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:14:28
0:14:28
 0:03:47
0:03:47
 0:03:59
0:03:59
 0:02:16
0:02:16
 0:03:46
0:03:46
 0:06:33
0:06:33
 0:04:27
0:04:27
 0:08:34
0:08:34
 0:00:53
0:00:53
 0:02:29
0:02:29
 0:17:08
0:17:08
 0:02:31
0:02:31
 0:00:34
0:00:34
 0:01:04
0:01:04
 0:02:10
0:02:10
 0:04:25
0:04:25
 0:04:11
0:04:11
 0:01:40
0:01:40
 0:00:24
0:00:24
 0:02:36
0:02:36
 0:01:45
0:01:45
 0:07:13
0:07:13
 0:00:06
0:00:06
 0:11:05
0:11:05