filmov
tv
GCSE Chemistry - Allotropes of Carbon - Diamond and Graphite #18

Показать описание
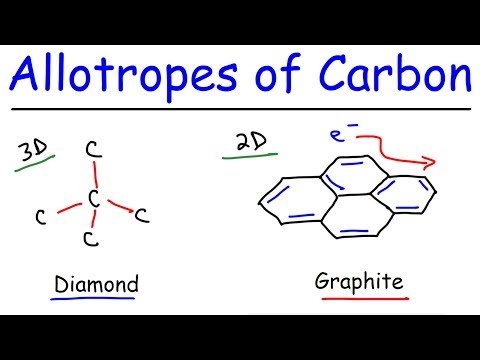
In this video, we explore the diamond and graphite which are two allotropes of solid carbon and we compare their structure and properties. Allotropes are different structural forms of the same element in the same physical state.
GCSE Chemistry - Allotropes of Carbon - Diamond and Graphite #18
GCSE Chemistry - Allotropes - Graphene and Fullerenes #19
Science Raps: GCSE Chemistry - Diamond and Carbon Allotropes
What Are Allotropes? Non-Metals | Properties of Matter | Chemistry | FuseSchool
Edexcel GCSE Chemistry - Allotropes of Carbon
GCSE Chemistry - Allotropes
Allotropes of Carbon
Allotropes of Carbon - Graphite, Diamond, Graphene, & Fullerenes
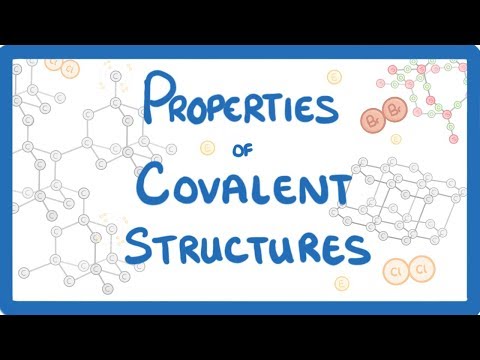
GCSE Chemistry - Properties of Simple Molecular Substances & Giant Covalent Structures #17
Bucky Balls, Nanotubes & Graphene | Organic Chemistry | Chemistry | FuseSchool
GCSE Chemistry 1-9: What is an Allotrope?
GCSE Chemistry Revision - (#16) Allotropes of Carbon
GCSE Science Revision (Chemistry): Allotropes of Carbon
Allotropes of Carbon | GCSE Science | Chemistry | Get To Know Science
Allotropes of Carbon AQA GCSE Edexcel IGCSE Chemistry
EVERYTHING YOU NEED TO KNOW ABOUT ALLOTROPES
Allotropes of Carbon | A-level Chemistry | OCR, AQA, Edexcel
GCSE Chemistry Carbon Allotropes Revision
GCSE Chemistry BONDING L6 - Allotropes of Carbon
GCSE Chemistry Allotropes of carbon
Giant Covalent Structures: Graphene and Fullerenes | 9-1 GCSE Chemistry | OCR, AQA, Edexcel
GCSE Chemistry - Metallic Bonding #20
GCSE Chemistry Revision - Allotropes of Carbon (Fullerenes & Graphene)
7 GCSE Allotropes of Carbon
Комментарии
 0:03:22
0:03:22
 0:03:07
0:03:07
 0:00:33
0:00:33
 0:04:37
0:04:37
 0:01:12
0:01:12
 0:00:16
0:00:16
 0:03:15
0:03:15
 0:06:33
0:06:33
 0:04:46
0:04:46
 0:04:52
0:04:52
 0:03:27
0:03:27
 0:06:40
0:06:40
 0:04:51
0:04:51
 0:05:11
0:05:11
 0:08:56
0:08:56
 0:00:57
0:00:57
 0:03:17
0:03:17
 0:02:47
0:02:47
 0:02:38
0:02:38
 0:02:22
0:02:22
 0:02:12
0:02:12
 0:03:31
0:03:31
 0:01:00
0:01:00
 0:04:04
0:04:04