filmov
tv
Chemistry Lec 3 | Kinetic Particle Theory | O Levels Pro

Показать описание
This lecture covers all the CIE objectives related to 'Kinetic Particle Theory', and also included are various types of past paper questions.
For queries/inquiries please refer to the following links:
Sir Atif Ilyas
+92 343 4669963
For queries/inquiries please refer to the following links:
Sir Atif Ilyas
+92 343 4669963
Chemistry Lec 3 | Kinetic Particle Theory | O Levels Pro
MDCAT I s and p Block Elements I Unit 11 I Lec # 3 I Prof. Wajid Ali Kamboh | WAK Entry Test
MDCAT I Gases I Unit 3 I Lec # 3 I Prof. Wajid Ali Kamboh | WAK Entry Test
MDCAT I Reaction Kinetics I Unit 7 I Lec # 3 I Prof. Wajid Ali Kamboh | WAK Entry Test
Chemical Kinetics lec-3 | Zero order reaction|CUET Domain Chemistry|CUET 2023 Free Class|Vaibhav Sir
Chemical Kinetics 03 : Rate Law and Order Of Reaction JEE MAINS/NEET
Chemical kinetics Lec-3 | Zero, First and Second order kinetics| CSIR-NET/JRF |GATE | IIT-JAM | DU|
'Chemical Kinetics LEC 03: Mastering Rate of Reaction for NEET/JEE 2025 | Hinglish | Arvind Sir...
Ch#9 |Lec#3 | Order Of Reaction and their Types |#Chemistry 11
Chemistry Class 12th/Chemical Kinetics / LEC 3
MDCAT I Gases I Unit 3 I Lec # 1 I Prof. Wajid Ali Kamboh | WAK Entry Test
Chemical Kinetics || Lec # 3 || Zero Order Reaction || First Order Reaction || Dr. Rizwana
MDCAT I Introduction of Fundamental Concepts of Chemistry I Unit 1 | Lec # 3 I WAK Entry Test
Chemistry | Chemical kinetics lec 3 | Rate law/Rate equation | Haresh Tutorial
MDCAT I Chemical Equilibrium I Unit 6 I Lec # 3 I Prof. Wajid Ali Kamboh | WAK Entry Test
Potential and kinetic energy - Law of conservation of energy - Video for kids
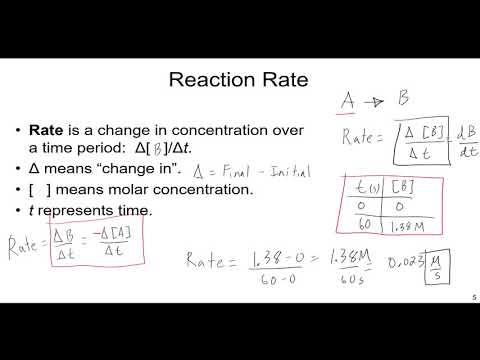
Kinetics: Initial Rates and Integrated Rate Laws
States of Matter - Solids, Liquids, Gases & Plasma - Chemistry
Potential and Kinetic Energy | #aumsum #kids #science #education #children
MDCAT I Chemical Equilibrium I Unit 6 I Lec # 2 I Prof. Wajid Ali Kamboh | WAK Entry Test
An Introduction to Chemical Kinetics
Chemical Kinetics Lec-2 | NEET 2024 | Free Crash Course For NEET
Degrees of freedom | Kinetic theory of gases | IIT JEE
Introduction to Electrochemistry
Комментарии
 0:22:27
0:22:27
 0:59:50
0:59:50
 0:48:24
0:48:24
 0:33:56
0:33:56
 1:05:08
1:05:08
 1:05:12
1:05:12
 0:55:00
0:55:00
 1:22:00
1:22:00
 0:26:50
0:26:50
 0:57:07
0:57:07
 1:07:06
1:07:06
 0:14:03
0:14:03
 0:56:06
0:56:06
 0:28:38
0:28:38
 0:41:53
0:41:53
 0:03:56
0:03:56
 0:09:10
0:09:10
 0:12:46
0:12:46
 0:04:28
0:04:28
 1:02:35
1:02:35
 0:25:24
0:25:24
 1:32:56
1:32:56
 0:03:03
0:03:03
 0:06:59
0:06:59