filmov
tv
Introduction to Electrochemistry

Показать описание
This lecture is about introduction to electrochemistry. I will teach you all the important concepts of electrochemistry.
#electrochemistry
#najamacademy
#chemistry
To learn more, watch this lecture till the end.
#electrochemistry
#najamacademy
#chemistry
To learn more, watch this lecture till the end.
Introduction to Electrochemistry
Introduction to Electrochemistry
Electrochemistry
Electrochemistry: Crash Course Chemistry #36
introduction to electrochemistry
Introduction to Electrochemistry
Matric part 1 Chemistry, Introduction About Electrochemistry - Ch 7 - 9th Class Chemistry
Basic introduction to Electrochemistry | ch#10 | 11th class Chemistry
A Level | Live Class 14 | Electrochemistry | Electrolysis of NaCl | WhatsApp +92 323 509 4443
Electrochemistry Practice Problems - Basic Introduction
Introduction to Electrochemistry
Introduction To Electrochemistry - Concept of Electrochemistry - Engineering Chemistry 2
Introduction to electrochemistry
Introduction to Electrochemistry and its Integration to In Situ TEM
Introduction to Electrochemistry | spontaneous and non spontaneous process | Electrochemistry
Introduction to Electrochemistry - Electrochemistry - Chemistry Class 12
20.1 Introduction to Electrochemistry
Introduction to Galvanic Cells & Voltaic Cells
An Introduction to #electrochemistry and ElectroReact | #webinar
17_01 Introduction to Electrochemistry
Introduction to Electrochemistry
Introduction to Electrochemistry (Part 1)
Chemistry | Electrochemistry | Galvanic cell (Full lesson)
Introduction to Electrochemistry
Комментарии
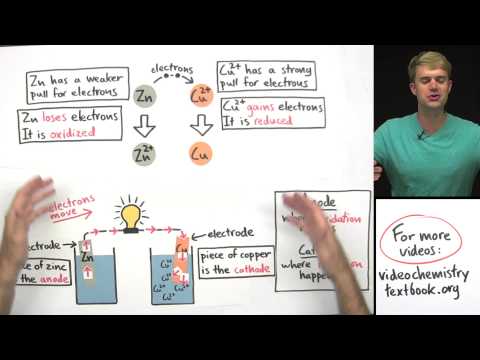 0:16:37
0:16:37
 0:06:59
0:06:59
 0:06:21
0:06:21
 0:09:04
0:09:04
 0:06:44
0:06:44
 0:43:01
0:43:01
 0:05:58
0:05:58
 0:28:06
0:28:06
 0:36:06
0:36:06
 0:53:39
0:53:39
 0:21:21
0:21:21
 0:11:01
0:11:01
 0:01:38
0:01:38
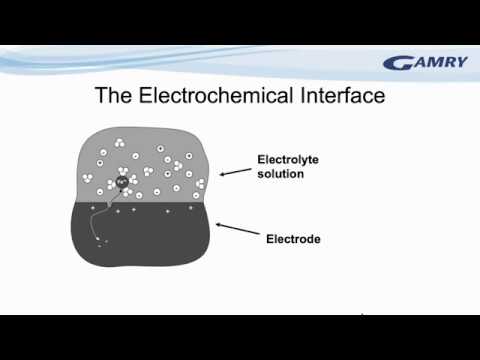 0:56:53
0:56:53
 0:17:56
0:17:56
 0:03:03
0:03:03
 0:09:14
0:09:14
 0:27:42
0:27:42
 0:43:34
0:43:34
 0:06:08
0:06:08
 0:20:48
0:20:48
 0:28:14
0:28:14
 0:56:52
0:56:52
 0:18:22
0:18:22