filmov
tv
Diels-Alder Cycloaddition

Показать описание
The mechanism and stereochemistry of the Diels-Alder reaction are examined here. This cycloaddition is one of the coolest reactions in organic chemistry. You'll see.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Diels-Alder Cycloaddition
Diels Alder Reaction
Pericyclic Reactions Part 1: Revisiting the Diels-Alder Reaction
The Diels Alder Cycloaddition
Diels-Alder cycloaddition basics
introduction to the Diels-Alder cycloaddition
Diels-Alder cycloaddition with Benzyne
Diels Alder Reaction Stereochemistry and Endo vs Exo Products
Diels-Alder Reaction || All TYPES || Cycloaddition || Pericyclic || Lecture3 || Tricky Example
Learn Chemistry: The Diels-Alder cycloaddition reaction
Diels-Alder Cycloaddition - Intrinsic Bond Orbital Visualization - Shiny Version
Diels-Alder Reaction|| [4+2] Cycloaddition reaction|| CSIR-NET|| GATE|| MSC Entrances.#shorts
Diels-Alder cycloaddition
Diels-Alder Cycloaddition
Module 4A, 1 introducing Diels Alder cycloaddition
Diels Alder Reaction Mechanism and Product Trick by Leah4sci
16.5 Diels-Alder Reactions | Organic Chemistry
The Diels-Alder Cycloaddition Reaction
Pericyclic Reactions Part 2: Hetero-DA Reactions and 1,3-Dipolar Cycloadditions
Diels-Alder Cycloaddition Animation
Pericyclic Reactions: The Diels-Alder Cycloaddition
The Diels-Alder & Other Pericyclic Reactions: Crash Course Organic Chemistry #42
Diel's-Alder Reaction ( Cyclo-addition reaction).
Diels-Alder reaction
Комментарии
 0:09:33
0:09:33
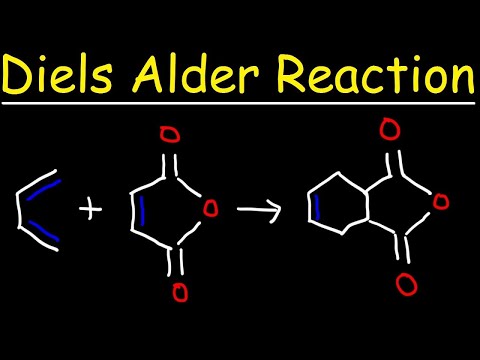 0:11:14
0:11:14
 0:10:14
0:10:14
 0:02:17
0:02:17
 0:02:40
0:02:40
 0:04:23
0:04:23
 0:01:34
0:01:34
 0:06:08
0:06:08
 0:24:32
0:24:32
 0:05:47
0:05:47
 0:00:06
0:00:06
![Diels-Alder Reaction|| [4+2]](https://i.ytimg.com/vi/gtyJ66z6d0c/hqdefault.jpg) 0:00:08
0:00:08
 0:05:10
0:05:10
 0:09:37
0:09:37
 0:08:16
0:08:16
 0:12:06
0:12:06
 0:46:11
0:46:11
 0:09:33
0:09:33
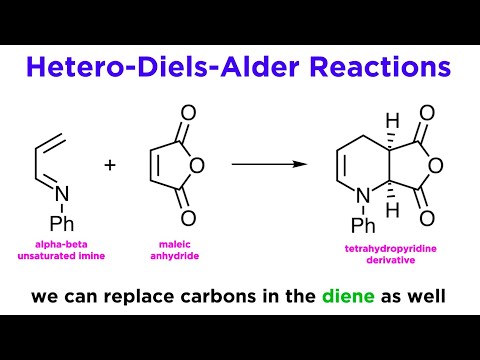 0:06:38
0:06:38
 0:00:09
0:00:09
 0:07:41
0:07:41
 0:13:01
0:13:01
 0:03:30
0:03:30
 0:11:20
0:11:20