filmov
tv
Diels Alder Reaction Stereochemistry and Endo vs Exo Products

Показать описание
This video gives you tips and tricks on how to draw the stereochemistry for Diels Alder products when substituents are present on both the diene and dienophile including stereochemistry for a bicyclic product. You’ll also learn how to distinguish between endo and exo products for dienophiles with both cis and trans configurations.
↪ Links & Resources Mentioned In This Video ↩
- - - - - - - - - - - - - - - - - - - - - - - -
⏱ In this video:
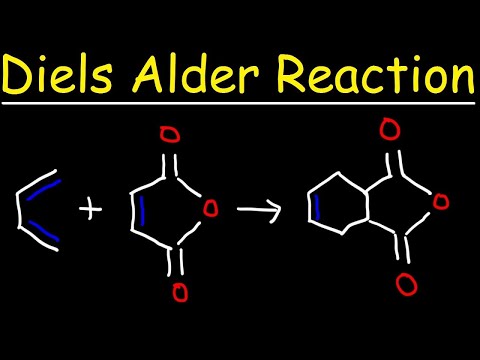
[0:16] Diels Alder Reaction Overview
[0:56] Why the Product Needs Stereochemistry
[1:17] Diene Stereochemistry
[2:52] Cyclic Diene Example
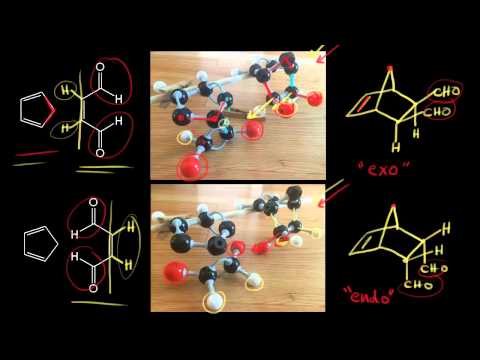
[3:46] Dienophile Stereochemistry - Endo and Exo
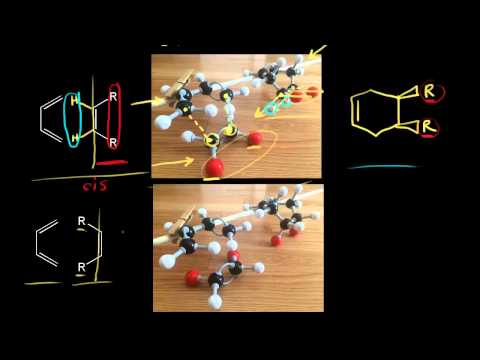
[4:34] Dienophile Stereochemistry - Cis and Trans
- - - - - - - - - - - - - - - - - - - - - - - -
🔔 Subscribe to my channel so you don’t miss out on any new videos 🔔
Diels Alder Reaction Stereochemistry and Endo vs Exo Products
Stereochemistry of the Diels-Alder Reaction
Diels Alder Reaction
Stereochemistry of Diels-Alder
Diels-Alder Cycloaddition
Pericyclic Reactions Part 1: Revisiting the Diels-Alder Reaction
Stereochemistry of the Diels-Alder reaction
The Alder Endo Rule and Diels-Alder Stereochemistry
Diels alder reaction stereochemistry|exo endo| mechanism|pericyclic reactions for CSIR-NET GATE
Diels-Alder reaction stereochemistry: the endo rule
Diels-Alder: endo rule | Organic chemistry | Khan Academy
diene & dienophile stereochemistry in the Diels-Alder
Diels-Alder: stereochemistry of diene | Organic chemistry | Khan Academy
Diels-Alder reaction stereochemistry: the diene
Diels-Alder: stereochemistry of dienophile | Organic chemistry | Khan Academy
Diels alder reaction stereochemistry| Examples | Organic chemistry| Pericyclic reactions| Carruthers
Endo and Exo Selectivity in the Diels-Alder Reaction
Diels-Alder: regiochemistry | Organic chemistry | Khan Academy
16.5 Diels-Alder Reactions | Organic Chemistry
16.5d Conservation of Orbital Symmetry in Diels Alder Reactions
Cycloaddition Reactions:Diels-Alder Reaction, Mechanism, Stereochemical Aspects,Exo & Endo Appro...
Diels Alder Reaction Mechanism and Product Trick by Leah4sci
Diels-Alder reaction | Organic chemistry | Khan Academy
Draw the product of each Diels-Alder reaction, and indicate the stereochemistry at all stereogenic …...
Комментарии
 0:06:08
0:06:08
 0:10:30
0:10:30
 0:11:14
0:11:14
 0:15:30
0:15:30
 0:09:33
0:09:33
 0:10:14
0:10:14
 0:11:08
0:11:08
 0:07:03
0:07:03
 0:29:57
0:29:57
 0:14:25
0:14:25
 0:09:56
0:09:56
 0:03:32
0:03:32
 0:10:37
0:10:37
 0:06:39
0:06:39
 0:08:48
0:08:48
 0:57:35
0:57:35
 0:05:37
0:05:37
 0:03:57
0:03:57
 0:46:11
0:46:11
 0:03:41
0:03:41
 0:35:25
0:35:25
 0:12:06
0:12:06
 0:08:02
0:08:02
 0:00:33
0:00:33