filmov
tv
Calculating final temperature through heat exchange in thermal equilibrium

Показать описание
This video helps you set up how to solve a problem in which we don't know the energy or the change in temperature Associated when two objects come into contact. We will briefly discuss system and surroundings and thermal equilibrium in order to solve how to find the final temperature when two objects at different temperatures come into contact.
Final Temperature Calorimetry Practice Problems - Chemistry
What is the Final Temperature given Heat (q=mcΔT)
Specific Heat (Solving for Final Temperature)
Calorimetry Examples: How to Find Heat and Specific Heat Capacity
Calculating final temperature through heat exchange in thermal equilibrium
Solving for temperature final in the Heat equation : Q=mc∆t
AP Specific Heat (Final Temp. Metal Dropped into Water)
Heat Capacity, Specific Heat, and Calorimetry
Unit 1 Physics Heat transfer 1 Conduction
How To Solve Basic Calorimetry Problems in Chemistry
Finding Final Temperature When Ice is Added to Water
Final Temperature of Ice and Water Mixture - How Many Grams of Ice Will Melt?
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial and Final Temperature, Mass
How to Calculate Specific Heat #chemistry #science #homework #shorts
Specific Heat Equation Stated Clearly
Specific Heat Capacity - Solving for Change in Temperature
Specific Heat Capacity - Solving for Initial Temperature
Solving for Final Temperature Using Specific Heat
AP Specific Heat (Final Temp. Ice Dropped into Water)
Thermodynamics: Specific Heat Capacity Calculations
Solving Heat Capacity and Specific Heat Capacity problems - Pure Physics
ALEKS: Using specific heat capacity to find temperature change
change in temperature calculations
(The Answer) Calculating the final temperature when two objects reach thermal equilibrium (Part 2)
Комментарии
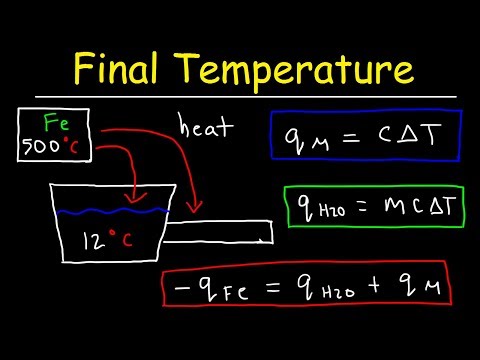 0:18:16
0:18:16
 0:02:44
0:02:44
 0:02:25
0:02:25
 0:04:13
0:04:13
 0:10:54
0:10:54
 0:11:57
0:11:57
 0:04:25
0:04:25
 0:04:14
0:04:14
 1:17:59
1:17:59
 0:10:25
0:10:25
 0:10:49
0:10:49
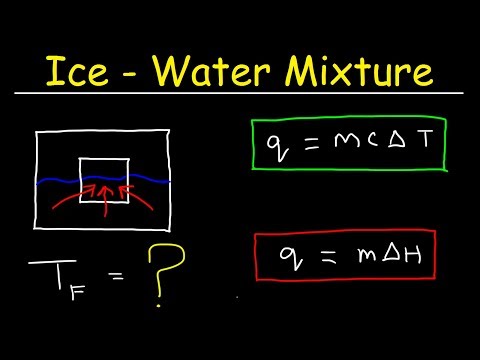 0:18:45
0:18:45
 0:09:19
0:09:19
 0:00:47
0:00:47
 0:03:56
0:03:56
 0:01:43
0:01:43
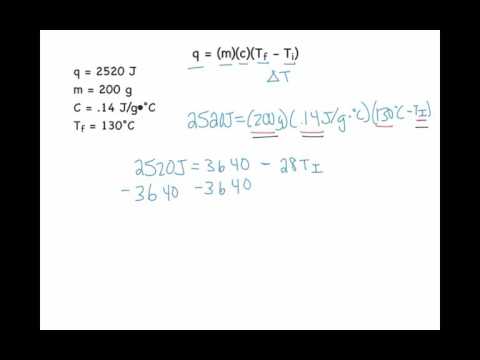 0:04:28
0:04:28
 0:07:18
0:07:18
 0:03:35
0:03:35
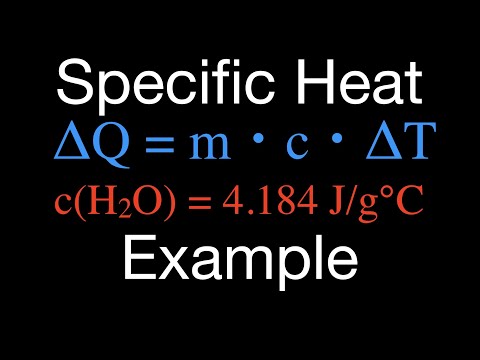 0:04:38
0:04:38
 0:03:53
0:03:53
 0:05:23
0:05:23
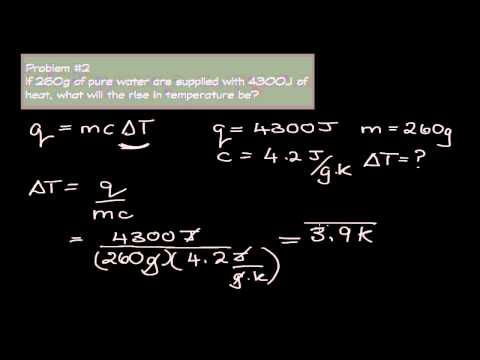 0:12:48
0:12:48
 0:15:09
0:15:09