filmov
tv
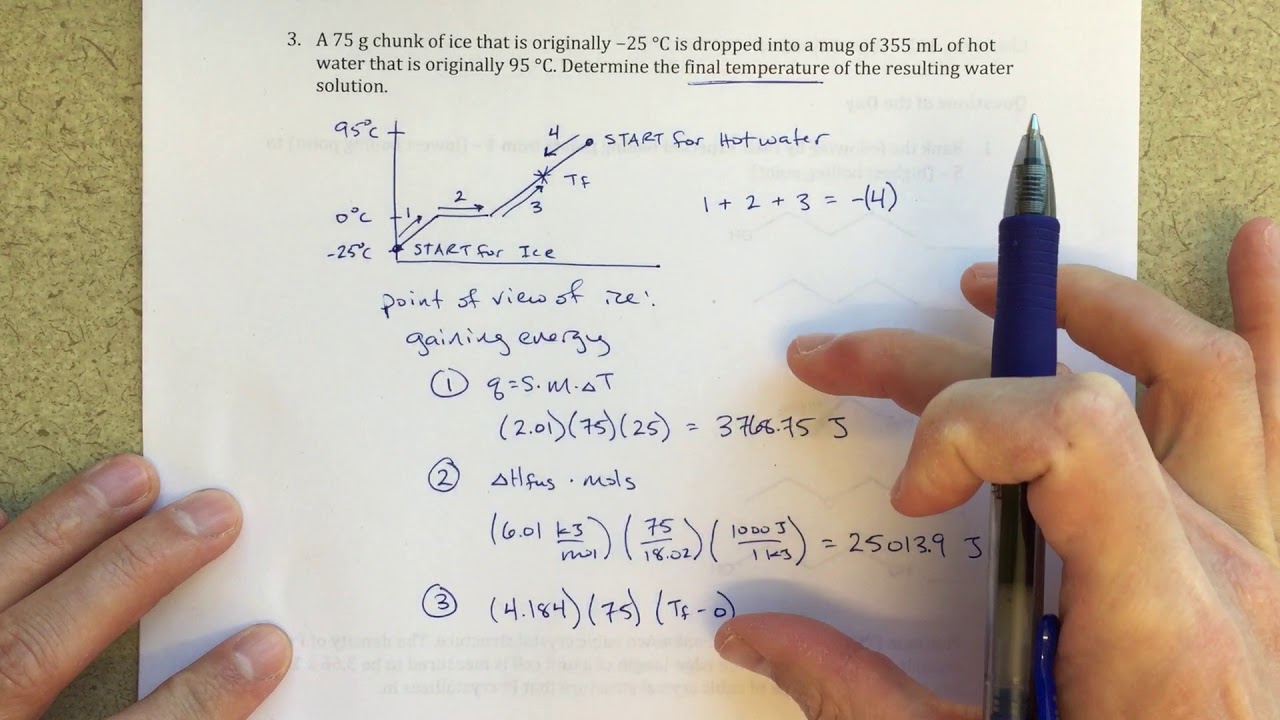
Finding Final Temperature When Ice is Added to Water
Показать описание
Finding Final Temperature When Ice is Added to Water
Final Temperature of Ice and Water Mixture - How Many Grams of Ice Will Melt?
Final Temperature Calorimetry Practice Problems - Chemistry
Ice in Water Final Temperature Problem
Steam at 100°C is added to ice at 0°C. (a) Find the amount of ice melted and the final temperature w...
what is the final temperature when ice is added to water?
AP Specific Heat (Final Temp. Ice Dropped into Water)
Complex Calorimetry II
UK's First Taste of Winter: Snow and Ice Alert! #UKWeather #shorts #WeatherUpdate #shortvideo
Cooling a Beverage - Enthalpy Calculation of adding ice to warm water
Thermodynamics: Calculating Latent and Specific Heat, Example Problem
Finding Final Temperature When Ice Water And Steam Are Mixed | Calorimetry
What will be the final temperature when 150 g of ice at `0^@C` is mixed with 300 g of water at `50^@
how much ice is needed to cool water? Calculation
Find the final temperature and composition of the mixture of \( 1 \mathrm{~kg} \) of ice at \( -...
Temperature and Heat: Calorimetry. Level 2, Example 2
Physics - Final temperature of a mixture - Thermal properties of matter - Part 5 - English
100 g ice at 0゚ is mixed with 10g steam at 100゚C find the final temperature and composition
Best NumeRiCaL of CaloRiMeTrY : Challenge !!
AP Specific Heat (Final Temp. Metal Dropped into Water)
What will be the final temperature when 150 g of ice at `0^C` is mixed with 300 g of water at `5...
THERMAL PROPERTIES 30g ice at 0C is mixed with 2g steam at100C The final temperature of the
Dry ice in water #Experiment #Science #Science #ASMR #DryIce
Calorimetry Examples: How to Find Heat and Specific Heat Capacity
Комментарии
 0:10:49
0:10:49
 0:18:45
0:18:45
 0:18:16
0:18:16
 0:11:17
0:11:17
 0:09:59
0:09:59
 0:06:59
0:06:59
 0:03:35
0:03:35
 0:08:03
0:08:03
 0:00:48
0:00:48
 0:17:25
0:17:25
 0:06:46
0:06:46
 0:00:52
0:00:52
 0:04:01
0:04:01
 0:08:38
0:08:38
 0:03:23
0:03:23
 0:04:21
0:04:21
 0:05:38
0:05:38
 0:18:52
0:18:52
 0:10:40
0:10:40
 0:04:25
0:04:25
 0:04:00
0:04:00
 0:05:49
0:05:49
 0:00:21
0:00:21
 0:04:13
0:04:13