filmov
tv
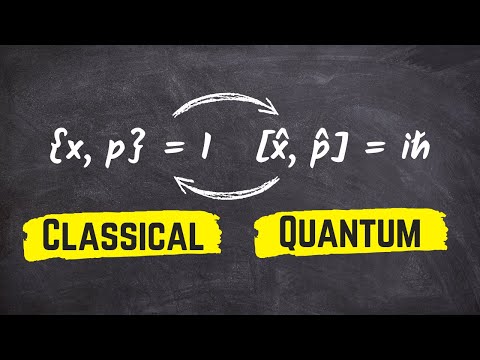
Difference Between Classical Mechanics and Quantum Mechanics

Показать описание
Quantum Chemistry Lecture 1:
What is Quantum Mechanics? Why classical mechanics failed? Applications of Quantum Mechanics
Quantum Chemistry Lecture 2:
Difference between Quantum Mechanics and Classical Mechanics
Quantum Chemistry Lecture 3:
Heisenberg Uncertainty Principle
Quantum Chemistry Lecture 4:
De Broglie Hypothesis | Wave and Particle nature of matter
Quantum Chemistry Lecture 5:
Applications and Significance of Schrodinger Wave Equation
Quantum Chemistry Lecture 6:
What is wave Function? What does it tell us? Born interpretation of wave Function | Probability of finding a particle
Quantum Chemistry Lecture 7:
Eigen Functions and Eigen values along with Examples
Quantum Chemistry Lecture 8:
What is *Normalization* of wave function? *Why* the wave function should be normalized? *Significance* of Normalization
*Numerical Problems* on Normalization of wave function
Quantum Chemistry Lecture 9:
*Orthogonality* of wave function and *orthonormal* wave function with *Numerical Problems*
Quantum Chemistry Lecture 10:
What are operators in Quantum Mechanics and How to use them?
Quantum Chemistry Lecture 11:
Algebra of Operators in Quantum Mechanics | Properties | Addition subtraction and Multiplication
Quantum Chemistry Lecture 12:
Commutative and Non Commutative Operators with solved Problems
Quantum Chemistry Lecture 13:
Rules for writing an operator
Quantum Chemistry Lecture 14:
Hermitian Operator in Quantum Mechanics
Quantum Chemistry Lecture 15:
Properties of Hermitian Operator
Quantum Chemistry Lecture 16:
Second Property:
*Eigen Functions of Hermitian Operator corresponding to different Eigen Values are orthogonal*
Quantum Chemistry Lecture 17:
Prove that the operator for linear momentum is Hermitian
Quantum Chemistry Lecture 18:
Prove that the kinetic Energy operator is Hermitian Operator
Quantum Chemistry Lecture 19:
if two operators are Hermitian then their product is also Hermitian if they commute
Quantum Chemistry Lecture 20:
Postulates of Quantum Mechanics
Quantum Chemistry Lecture 21:
Time independent Schrodinger wave equation
Quantum Chemistry Lecture 22:
Steady state schrodinger wave equation
Quantum Chemistry Lecture 23:
Conclusion of particle in one dimensional box:
Quantum Chemistry Lecture 24:
Conclusion of particle in one dimensional box
What is Quantum Mechanics? Why classical mechanics failed? Applications of Quantum Mechanics
Quantum Chemistry Lecture 2:
Difference between Quantum Mechanics and Classical Mechanics
Quantum Chemistry Lecture 3:
Heisenberg Uncertainty Principle
Quantum Chemistry Lecture 4:
De Broglie Hypothesis | Wave and Particle nature of matter
Quantum Chemistry Lecture 5:
Applications and Significance of Schrodinger Wave Equation
Quantum Chemistry Lecture 6:
What is wave Function? What does it tell us? Born interpretation of wave Function | Probability of finding a particle
Quantum Chemistry Lecture 7:
Eigen Functions and Eigen values along with Examples
Quantum Chemistry Lecture 8:
What is *Normalization* of wave function? *Why* the wave function should be normalized? *Significance* of Normalization
*Numerical Problems* on Normalization of wave function
Quantum Chemistry Lecture 9:
*Orthogonality* of wave function and *orthonormal* wave function with *Numerical Problems*
Quantum Chemistry Lecture 10:
What are operators in Quantum Mechanics and How to use them?
Quantum Chemistry Lecture 11:
Algebra of Operators in Quantum Mechanics | Properties | Addition subtraction and Multiplication
Quantum Chemistry Lecture 12:
Commutative and Non Commutative Operators with solved Problems
Quantum Chemistry Lecture 13:
Rules for writing an operator
Quantum Chemistry Lecture 14:
Hermitian Operator in Quantum Mechanics
Quantum Chemistry Lecture 15:
Properties of Hermitian Operator
Quantum Chemistry Lecture 16:
Second Property:
*Eigen Functions of Hermitian Operator corresponding to different Eigen Values are orthogonal*
Quantum Chemistry Lecture 17:
Prove that the operator for linear momentum is Hermitian
Quantum Chemistry Lecture 18:
Prove that the kinetic Energy operator is Hermitian Operator
Quantum Chemistry Lecture 19:
if two operators are Hermitian then their product is also Hermitian if they commute
Quantum Chemistry Lecture 20:
Postulates of Quantum Mechanics
Quantum Chemistry Lecture 21:
Time independent Schrodinger wave equation
Quantum Chemistry Lecture 22:
Steady state schrodinger wave equation
Quantum Chemistry Lecture 23:
Conclusion of particle in one dimensional box:
Quantum Chemistry Lecture 24:
Conclusion of particle in one dimensional box
Комментарии
 0:08:21
0:08:21
 0:05:16
0:05:16
 0:14:20
0:14:20
 0:01:22
0:01:22
 0:01:00
0:01:00
 0:00:52
0:00:52
 0:01:42
0:01:42
 0:00:31
0:00:31
 0:13:57
0:13:57
 0:00:35
0:00:35
 0:06:08
0:06:08
 0:18:33
0:18:33
 0:17:15
0:17:15
 0:00:31
0:00:31
 0:01:00
0:01:00
 0:01:00
0:01:00
 0:26:31
0:26:31
 0:07:47
0:07:47
 0:00:55
0:00:55
 0:00:27
0:00:27
 0:11:05
0:11:05
 0:10:05
0:10:05
 0:00:41
0:00:41
 0:00:06
0:00:06