filmov
tv
Chemical Equations | Environmental Chemistry | Chemistry | FuseSchool

Показать описание
Chemical equation shows the overall chemical change of reactants into products. It's a bit like a detailed cooking recipe, but where all the ingredients and all the products are written down, even the ones you can't necessarily see.
The reactants are what you start with, and the products are what are formed. There are two ways of writing chemical equations; word equations and symbol equations. when they are written, both types show the reactant on the left of an arrow and the products on the right. The arrow is there to show that the reaction is irreversible. If you like, it shows the direction of the reaction and that it is one way. A bit like a one-way street. You can't reverse along a one-way street and you can't reverse an irreversible reaction.
Let's look at the word equation for neutralising hydrochloric acid with sodium hydroxide. This is a way of summarising a chemical reaction. The plus sign indicates that there is more than one reactant or product on each side of the equation, with the reactants on the left and the products on the right. Note that we've also written this all on a single line. If you can't keep an equation on one line, then the arrow becomes an important separator and the rule 'the reactants on the left and products on the right ' still applies , because if not, the equation can become a jumbled mess.
A clearly written equation is always easier to understand. A word equation provides a good summary, but a symbol equation provides more information. It shows more detail and allows us to see how many atoms and molecules are evolved in each reaction. The little letters in brackets are state symbols . they show this state of matter of each product and reactant and this is covered in ore detail in our lesson 'State symbols in chemical equations'.
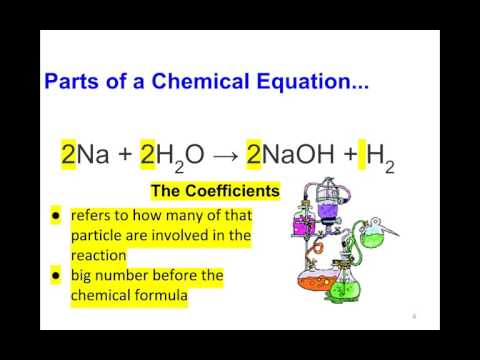
There are occasions where a reaction is reversible due to the changes in the surroundings . For example - pressure , concentration , pH and temperature. And where this is the case, we draw a double arrow made of two half arrows pointing in opposite directions. And this indicates that the reaction can go either way. The formation of ammonia from nitrogen and hydrogen is an example of a reversible reaction, and a key thing here is that you recognize reversible reaction by the double arrows. And you'll notice some numbers in front of the hydrogen and ammonia formulae and these are there to make the equation balance. you can find out more about this in our 'balancing equations 'lesson.
So to summarise, a chemical equation shows the overall chemical change of reactants into products. We usually write reactants on the left of the arrow and products on the right. A single arrow means that the reaction is irreversible and two oppositely pointing half arrows means the reaction is reversible .
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
Find all of our Biology videos here:
Find all of our Maths videos here:
The reactants are what you start with, and the products are what are formed. There are two ways of writing chemical equations; word equations and symbol equations. when they are written, both types show the reactant on the left of an arrow and the products on the right. The arrow is there to show that the reaction is irreversible. If you like, it shows the direction of the reaction and that it is one way. A bit like a one-way street. You can't reverse along a one-way street and you can't reverse an irreversible reaction.
Let's look at the word equation for neutralising hydrochloric acid with sodium hydroxide. This is a way of summarising a chemical reaction. The plus sign indicates that there is more than one reactant or product on each side of the equation, with the reactants on the left and the products on the right. Note that we've also written this all on a single line. If you can't keep an equation on one line, then the arrow becomes an important separator and the rule 'the reactants on the left and products on the right ' still applies , because if not, the equation can become a jumbled mess.
A clearly written equation is always easier to understand. A word equation provides a good summary, but a symbol equation provides more information. It shows more detail and allows us to see how many atoms and molecules are evolved in each reaction. The little letters in brackets are state symbols . they show this state of matter of each product and reactant and this is covered in ore detail in our lesson 'State symbols in chemical equations'.
There are occasions where a reaction is reversible due to the changes in the surroundings . For example - pressure , concentration , pH and temperature. And where this is the case, we draw a double arrow made of two half arrows pointing in opposite directions. And this indicates that the reaction can go either way. The formation of ammonia from nitrogen and hydrogen is an example of a reversible reaction, and a key thing here is that you recognize reversible reaction by the double arrows. And you'll notice some numbers in front of the hydrogen and ammonia formulae and these are there to make the equation balance. you can find out more about this in our 'balancing equations 'lesson.
So to summarise, a chemical equation shows the overall chemical change of reactants into products. We usually write reactants on the left of the arrow and products on the right. A single arrow means that the reaction is irreversible and two oppositely pointing half arrows means the reaction is reversible .
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
Find all of our Biology videos here:
Find all of our Maths videos here:
Комментарии
 0:04:19
0:04:19
 0:18:49
0:18:49
 0:03:35
0:03:35
 0:14:56
0:14:56
 0:03:56
0:03:56
 0:05:18
0:05:18
 0:05:43
0:05:43
 0:21:56
0:21:56
 0:03:46
0:03:46
 0:03:15
0:03:15
 0:00:30
0:00:30
 0:03:56
0:03:56
 0:05:37
0:05:37
 0:04:12
0:04:12
 0:04:12
0:04:12
 0:03:25
0:03:25
 0:03:03
0:03:03
 0:05:41
0:05:41
 0:10:13
0:10:13
 0:04:33
0:04:33
 0:04:15
0:04:15
 0:04:22
0:04:22
 0:03:52
0:03:52
 0:00:31
0:00:31