filmov
tv
Quantum Chemistry 4.1 - Postulates of Quantum Mechanics 1: Wavefunctions (Old Version)

Показать описание
Quantum Chemistry 4.1 - Postulates of Quantum Mechanics 1: Wavefunction
Quantum Chemistry 4.1 - Postulates of Quantum Mechanics 1: Wavefunctions (Old Version)
What are the Postulates of Quantum Mechanics - Basic Quantum Chemistry
Quantum Chemistry 4.4 - Postulates of Quantum Mechanics 4: Expectation Values
Postulates of Quantum Mechanics Part l
Postulates of quantum mechanics
Chapter 4: Postulate 1 | CHM 309 | 037
Quantum Chemistry: Class - 4: Postulate - I (State of a Physical System)
Quantum Chemistry 4.2 - Postulates of Quantum Mechanics 2: Operators
Quantum Chemistry 4.4 - Postulates of Quantum Mechanics 4: Expectation Values (Old Version)
The Schrödinger Equation Explained in 60 Seconds
Postulates of Quantum mechanics & Explanation°QUANTUM CHEMISTRY°MSc Chemistry@itschemistrytime
Quantum Chemistry 4.3 - Postulates of Quantum Mechanics 3: Measurement
Postulates of Quantum chemistry// Quantum chemistry ⚗️
Postulate 's of quantum mechanics || Postulate 's of quantum mechanics
Postulate 4 of quantum mechanics | MSc Chemistry Sem 1 | University of Kerala
This chapter closes now, for the next one to begin. 🥂✨.#iitbombay #convocation
Postulates of Quantum Mechanics
Postulates of Quantum of Mechanics | Wave function | Eigen value | Expectation value | Operator
Postulate 4 (Part 1) of Quantum Mechanics
Trick to Calculate Atomic Mass of first 20 Elements #shorts #reels #chemistry
Postulates Of Quantum Mechanics #shorts #chemistry #quantum #bsc #jam #msc #viral #notes
Brian Cox explains quantum mechanics in 60 seconds - BBC News
Postulates (Part- 1) | Quantum Chemistry | Target IIT-JAM 2021 | Aman Rastogi
Комментарии
 0:04:57
0:04:57
 0:08:47
0:08:47
 0:02:02
0:02:02
 0:05:53
0:05:53
 0:29:12
0:29:12
 0:08:53
0:08:53
 0:07:16
0:07:16
 0:28:02
0:28:02
 0:03:59
0:03:59
 0:07:33
0:07:33
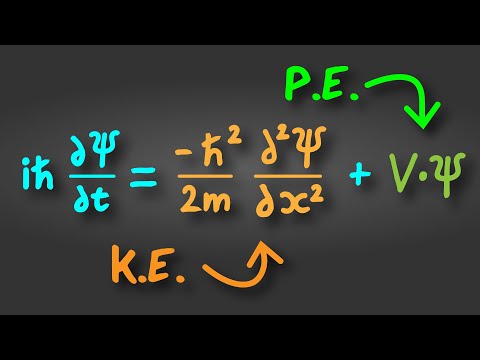 0:01:00
0:01:00
 0:33:47
0:33:47
 0:02:34
0:02:34
 0:00:18
0:00:18
 0:00:05
0:00:05
 0:40:09
0:40:09
 0:00:16
0:00:16
 0:28:43
0:28:43
 0:00:08
0:00:08
 0:14:55
0:14:55
 0:00:54
0:00:54
 0:00:12
0:00:12
 0:01:22
0:01:22
 1:24:05
1:24:05