filmov
tv
AP Chemistry Rate of Reactions Lesson 7 - Reaction Mechanisms, elementary step and fast equilibriums

Показать описание
In this final lesson of the AP Rate of Reaction unit, we will study what occurs between the beginning and end of a reaction. We will break down reactions into a series of elementary steps. The slowest step will correspond to the orders in the rate law.
00:00 Mechanism Intro/Intermediates
7:55 Uni/bi/termolecular
9:36 Chemical Potential Energy Diagrams
12:25 Catalyzed Peroxide Reaction
16:20 Photosynthesis Mechanism
18:35 Rate Laws without intermediates (fast equilibrium)
26:20 Practice Problem/Answer
32:40 Homework/Practice
00:00 Mechanism Intro/Intermediates
7:55 Uni/bi/termolecular
9:36 Chemical Potential Energy Diagrams
12:25 Catalyzed Peroxide Reaction
16:20 Photosynthesis Mechanism
18:35 Rate Laws without intermediates (fast equilibrium)
26:20 Practice Problem/Answer
32:40 Homework/Practice
 0:09:10
0:09:10
 0:10:09
0:10:09
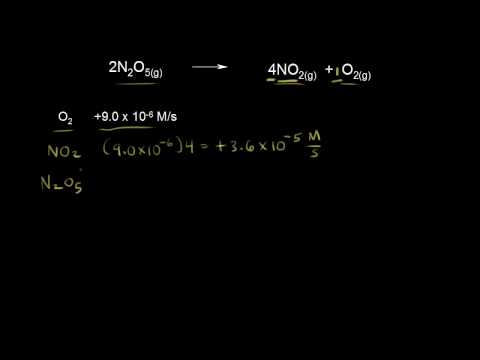 0:09:14
0:09:14
 0:19:13
0:19:13
 0:10:56
0:10:56
 0:07:18
0:07:18
 0:01:58
0:01:58
 0:18:48
0:18:48
 0:02:49
0:02:49
 0:10:39
0:10:39
 0:03:42
0:03:42
 0:48:46
0:48:46
 0:09:57
0:09:57
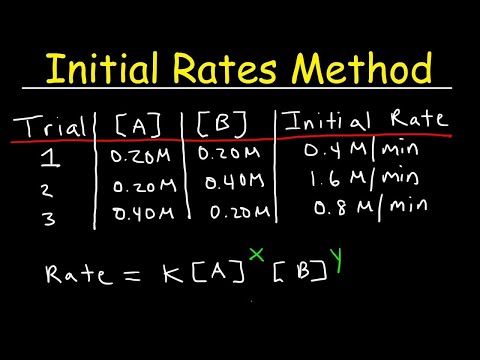 0:34:53
0:34:53
 0:09:39
0:09:39
 0:07:43
0:07:43
 0:09:41
0:09:41
 0:13:42
0:13:42
 0:08:42
0:08:42
 0:11:50
0:11:50
 0:06:48
0:06:48
 0:14:02
0:14:02
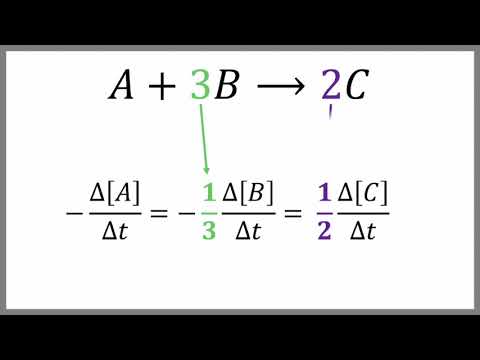 0:05:30
0:05:30
 0:05:08
0:05:08