filmov
tv
5 1b Reaction Rates and Stoichiometry

Показать описание
5 1b Reaction Rates and Stoichiometry
Chem 1B-Lec 5-Kinetics Part 1 (Rate Expression)
Chemical Kinetics - Initial Rates Method
DSE Chem 2023 Paper 1B (LQ) Q7 (Section 5)
Chem 1B - Week 9: Reaction Rates.
Chemistry - Chemical Kinetics (2 of 30) Reaction Rate- Definition
How to Find the Rate Law and Rate Constant (k)
MHA Characters Reacting to Ships but it’s kinda accurate || Gacha Club
Average and Instantaneous Rate of a chemical reaction | Chemical Kinetics | Chemistry | Khan Academy
CHEM 1B Unit 13c2 Kinetics
Chem 1B-Lec 9-Kinetics Part 5 (Activation Energy)
CHEM 1B Unit 13b1 Kinetics
Chem 1B-Lec 14-Kinetics Part 10 (Kinetics Review Session)
DSE Chem 2020 Paper 1B (LQ) Q8 (Section 5)
Chem 1B-Lec 6-Kinetics Part 2 (Rate Law)
Ansonia teen one of three in world to earn perfect score on AP Chemistry exam
Drawing a tangent to find instantaneous rate of reaction
CHEM 1B Unit 13a1 Kinetics
Chem 1B-Lec 8-Kinetics Part 4 (Integrated Rate Law)
Chemistry 51B: Organic Chemistry. Lecture 10
DSE Chem 2022 Paper 1B (LQ) Q6 (Section 5)
Chemistry 51B: Organic Chemistry. Lecture 2
Chemistry 51B: Organic Chemistry. Lecture 11
PS1B - Chemical Reactions
Комментарии
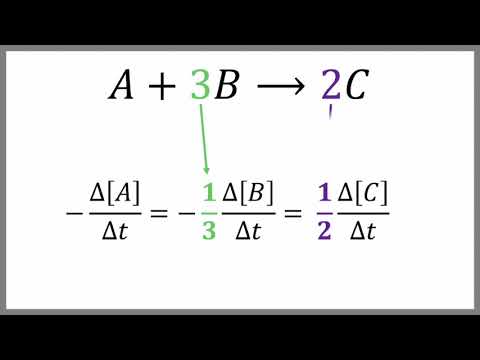 0:05:30
0:05:30
 0:28:42
0:28:42
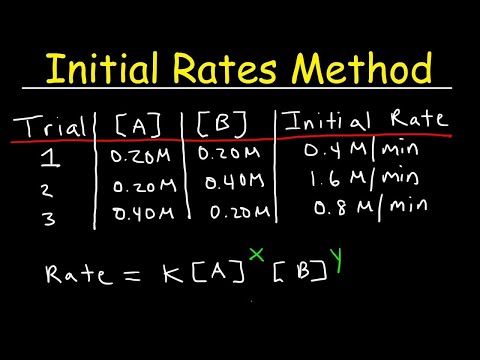 0:34:53
0:34:53
 0:08:11
0:08:11
 0:04:31
0:04:31
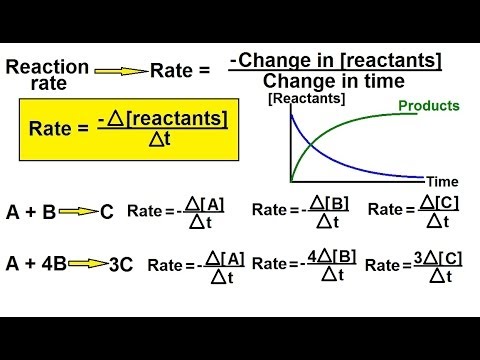 0:05:35
0:05:35
 0:03:42
0:03:42
 0:03:00
0:03:00
 0:12:29
0:12:29
 0:38:12
0:38:12
 0:27:33
0:27:33
 0:21:32
0:21:32
 0:47:14
0:47:14
 0:11:09
0:11:09
 0:27:02
0:27:02
 0:01:55
0:01:55
 0:05:30
0:05:30
 0:21:22
0:21:22
 0:29:15
0:29:15
 0:48:35
0:48:35
 0:03:35
0:03:35
 0:47:17
0:47:17
 0:46:42
0:46:42
 0:07:29
0:07:29