filmov
tv
Calculating [OH-] from PH

Показать описание
Calculating [OH-] from PH
PH = - log[OH-]
problems based on PH
In the given problem [OH-] concentration is given calculate POH then calculate PH
PH + POH = 14
↓↓↓↓ JMR’S CHEMISTRY ↓↓↓↓↓↓↓↓↓↓↓↓↓↓↓↓
Atoms, Molecules and Chemical Reactions
Electro chemistry
YouTube live videos
Chemical Equilibrium
Molarity
IIT Previous Years Questions
Organic chemistry
Gaseous state
Is matter around us
Hydrogen
Chemistry Practical
Periodic Table
Atomic structure
Symbols and formula
Percentage Composition
Mole concept
Chemical formula
Percentage yield
Acids and Bases
Metallurgy
Chemical bonding
Water / Solutions
PH = - log[OH-]
problems based on PH
In the given problem [OH-] concentration is given calculate POH then calculate PH
PH + POH = 14
↓↓↓↓ JMR’S CHEMISTRY ↓↓↓↓↓↓↓↓↓↓↓↓↓↓↓↓
Atoms, Molecules and Chemical Reactions
Electro chemistry
YouTube live videos
Chemical Equilibrium
Molarity
IIT Previous Years Questions
Organic chemistry
Gaseous state
Is matter around us
Hydrogen
Chemistry Practical
Periodic Table
Atomic structure
Symbols and formula
Percentage Composition
Mole concept
Chemical formula
Percentage yield
Acids and Bases
Metallurgy
Chemical bonding
Water / Solutions
 0:13:50
0:13:50
 0:04:05
0:04:05
 0:08:38
0:08:38
 0:10:52
0:10:52
![Calculating [OH-] from](https://i.ytimg.com/vi/hSTMWGLgH3M/hqdefault.jpg) 0:11:16
0:11:16
![calculating [H+] &](https://i.ytimg.com/vi/oRCDr7x9QQo/hqdefault.jpg) 0:05:07
0:05:07
 0:03:48
0:03:48
 0:12:41
0:12:41
 0:24:59
0:24:59
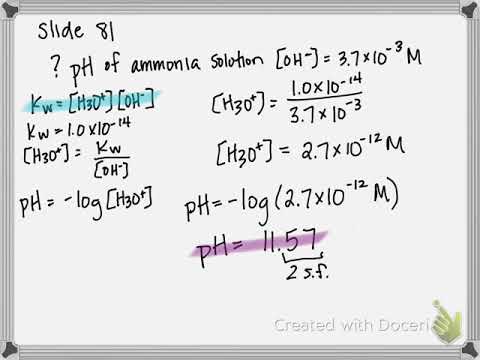 0:05:42
0:05:42
 0:09:14
0:09:14
 0:01:57
0:01:57
 0:01:31
0:01:31
 0:11:23
0:11:23
 0:04:35
0:04:35
 0:03:48
0:03:48
 0:02:02
0:02:02
![Calculating [H3O+] from](https://i.ytimg.com/vi/TOIeMRuRU08/hqdefault.jpg) 0:02:02
0:02:02
 0:08:07
0:08:07
 0:01:33
0:01:33
![Calculating [OH-] in](https://i.ytimg.com/vi/I2-V3fyo8Ps/hqdefault.jpg) 0:04:01
0:04:01
 0:00:46
0:00:46
 0:29:31
0:29:31
 0:12:03
0:12:03