filmov
tv
Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice
Показать описание
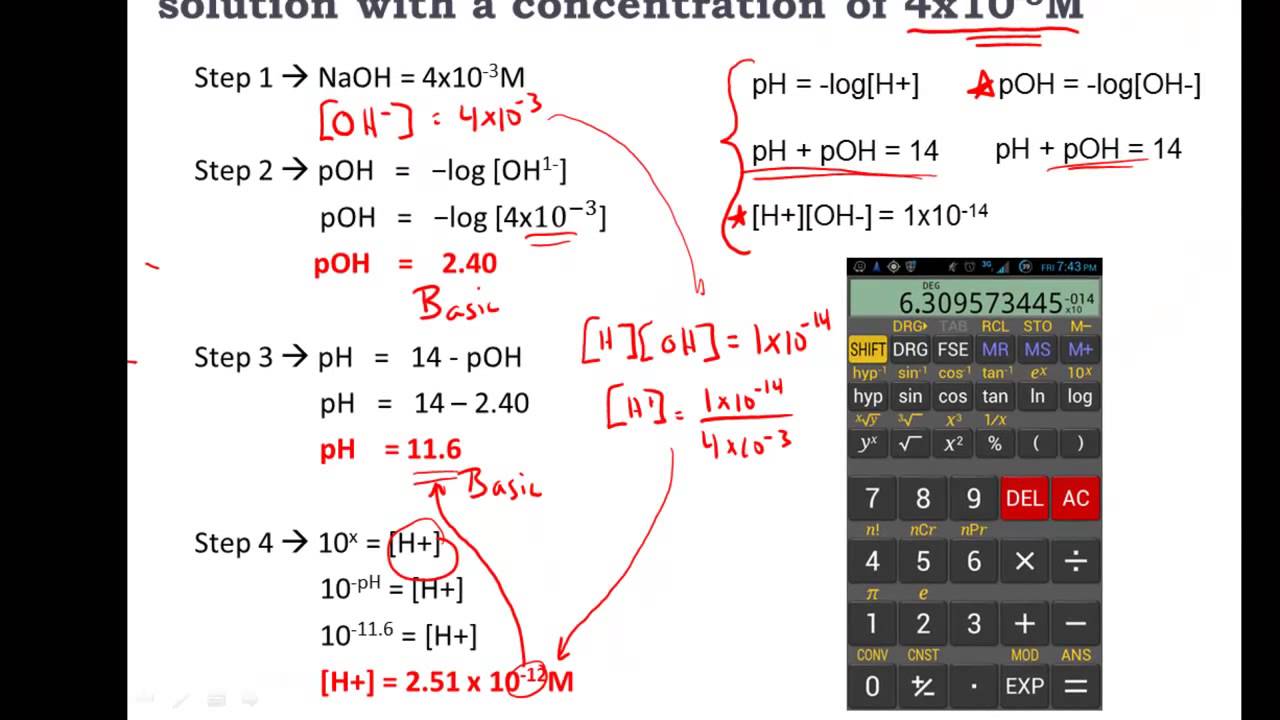
This video on acids and bases shows you how to calculate the pH, pOH, [H+], [OH-] of acid and base solutions. Acids and bases can be measured by their strength of pH and pOH. Tune in on this awesome acid base video.
Комментарии

















![pH, pOH, [H3O+],](https://i.ytimg.com/vi/AZlbgmYfYoE/hqdefault.jpg)





![pH, pOH, [H3O+],](https://i.ytimg.com/vi/BxX1NSWCFFE/hqdefault.jpg)