filmov
tv
How to find concentration of H+ given pH

Показать описание
For video requests or tutorials regarding math or chemistry feel free to message me! Also like and subscribe to keep the videos coming!
Concentration Formula & Calculations | Chemical Calculations | Chemistry | Fuse School
Concentration | How to Calculate Concentration of a Solution?
GCSE Chemistry - How to Calculate Concentration in grams per decimetre cubed #30
GCSE Chemistry - Moles, Concentration & Volume Calculations #29
Ion Concentration in Solutions From Molarity, Chemistry Practice Problems
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
Concentration grade 10
Concentration and Molarity explained: what is it, how is it used + practice problems
(40Hz) Gamma Binaural Beats | Meditation Music for Study, Concentration & Focus
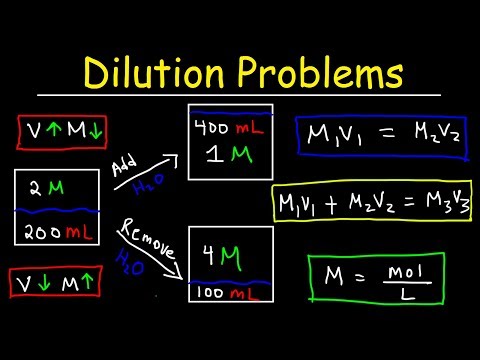
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
How to calculate concentration from pH and pOH
Parts Per Million (ppm) and Parts Per Billion (ppb) - Solution Concentration
How to Calculate Concentration of a Solution | Is Matter Around Us Pure| CBSE Class 9 Chemistry Ch-2
How to find concentration of H+ given pH
Concentration of a Solution | Chemistry
How to solve percent concentration problems even if you're 🤷🏻♀️ - Dr K
Calculating The Concentration #shorts
Concentration of solutions Chemistry
Calculating Molarity | Molar Concentration #chemistry #science #homework #shorts #short #education
Worked example: Calculating concentration using the Beer–Lambert law | AP Chemistry | Khan Academy
How to calculate the concentration of solution?
How To Calculate The Hydroxide Ion Concentration | Chemistry
Mass-Volume Percent: How to Solve Concentration Questions %(m/v)
How to Calculate Hydrogen Ion Concentration from pH
Комментарии
 0:04:25
0:04:25
 0:05:29
0:05:29
 0:03:28
0:03:28
 0:06:04
0:06:04
 0:12:24
0:12:24
 0:31:25
0:31:25
 0:23:41
0:23:41
 0:05:41
0:05:41
 9:09:10
9:09:10
 0:21:55
0:21:55
 0:03:14
0:03:14
 0:11:00
0:11:00
 0:02:13
0:02:13
 0:03:48
0:03:48
 0:03:21
0:03:21
 0:05:51
0:05:51
 0:00:41
0:00:41
 0:09:27
0:09:27
 0:00:58
0:00:58
 0:03:48
0:03:48
 0:05:22
0:05:22
 0:12:32
0:12:32
 0:06:51
0:06:51
 0:01:57
0:01:57