filmov
tv
Calcium oxide and water CaO + H2O EXPERIMENT

Показать описание
Calcium hydroxide (slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. The colorless crystals and has a white powder. Obtained by mixing calcium oxide (lime) with water.
Calcium hydroxide is produced industrially by treating lime with water:
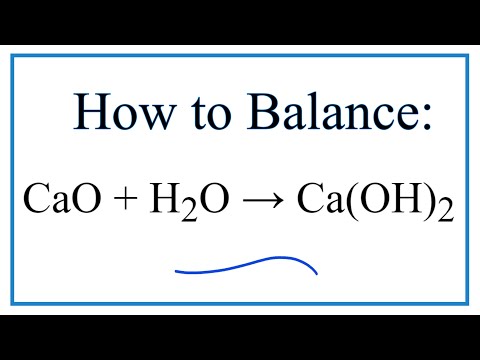
CaO + H2O → Ca(OH)2
Calcium hydroxide is produced industrially by treating lime with water:
CaO + H2O → Ca(OH)2
Calcium oxide and water CaO + H2O EXPERIMENT
Calsium Oxide(CaO) +Water H2O=Calsium Hydroxide Ca(OH)2 #experiment #reaction #like #subscribe
Reaction of Calcium oxide with water | Exothermic Reaction | Chemistry Demo
Don't Put Water on Chalk—Quicklime
Reaction of Calcium oxide (Quicklime) with water | Exothermic Reaction | CaO + H2O EXPERIMENT
Exothermic reaction | CaO + Water | #Class9&10 | Fun experiment🔥🔥🔥🔥
Experiment with CaO(Calcium Oxide) and Water.Watch this video till the end.
Quicklime and Water Reaction
How to Balance CaO + H2O = Ca(OH)2 (Calcium oxide plus Water)
Calcium Oxide (Chuna) and DiHydrogen Oxide (Water) Reaction | Chuna Pani | Exothermic Reaction
Using Chalk to Make Limelight!
Quick Lime (Calcium oxide) and Water react to form Calcium hydroxide | Combination Reaction
calcium oxide reacts with water
Calcium Hydroxide Experiment
Calcium is crazy
Calcium Oxide (CaO) Experiment . what is Quick Lime? Slaked lime? #Quicklime #experiment #science
Calcium Oxide and Water Experiment || Cao+H²o=Ca(OH)² Pratical
Reaction #4 - Calcium Oxide + Water
Limestone Cycle - limestone, quicklime and slaked lime | Chemistry | FuseSchool
Mixing calcium oxide with water 🔥😅 #shorts
Calcium Oxide | Cao | reaction with water
Co2 Test, Ca(OH)2 + CO2 → CaCO3 + H2O
Heat Calcium carbonate #shorts #experiment
Calcium oxide + water phenolphthalein
Комментарии
 0:01:48
0:01:48
 0:00:22
0:00:22
 0:03:18
0:03:18
 0:03:25
0:03:25
 0:04:01
0:04:01
 0:01:00
0:01:00
 0:03:21
0:03:21
 0:01:31
0:01:31
 0:01:19
0:01:19
 0:00:11
0:00:11
 0:00:36
0:00:36
 0:03:14
0:03:14
 0:00:40
0:00:40
 0:00:14
0:00:14
 0:01:00
0:01:00
 0:00:42
0:00:42
 0:06:59
0:06:59
 0:03:21
0:03:21
 0:04:32
0:04:32
 0:00:14
0:00:14
 0:00:29
0:00:29
 0:01:00
0:01:00
 0:00:20
0:00:20
 0:01:00
0:01:00