filmov
tv
Limestone Cycle - limestone, quicklime and slaked lime | Chemistry | FuseSchool

Показать описание
Limestone Cycle - limestone, quicklime and slaked lime | Chemistry | FuseSchool
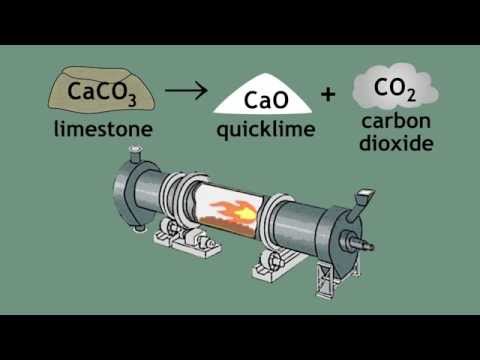
Learn the basics about limestone cycle - limestone, quicklime and slaked lime. What are their properties, similarities and differences?
Find out more in this video!
CREDITS
Design and animation: Marija Popova
Narration: Amanda Edward
Script: Amanda Edward
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Learn the basics about limestone cycle - limestone, quicklime and slaked lime. What are their properties, similarities and differences?
Find out more in this video!
CREDITS
Design and animation: Marija Popova
Narration: Amanda Edward
Script: Amanda Edward
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Limestone Cycle - limestone, quicklime and slaked lime | Chemistry | FuseSchool
Limestone Cycle limestone, quicklime and slaked lime
The Limestone Cycle
The Limestone Cycle: A Fascinating Chemistry Experiment
What Is The Limestone Cycle? | Environmental Chemistry | Chemistry | FuseSchool
Quicklime and Water Reaction
quicklime from limestone 2
The Lime Cycle Experiment
| lime cycle | limestone | quicklime | slakedlime |chemistry notes
Limestone Cycle Demo with SMI
Lime Cycle
The Limestone Cycle
Pure lime as mineral binder
Limestone
The Science Show E1 - The Limestone Cycle
Decomposition of calcium carbonate
Lab Demo: Hydrochloric Acid Reacting with Limestone
Primitive Technology: Lime
Quicklime and Water Reaction | Cornish Lime
making limestone
Limestone Reclaimer #youtubeshorts #shorts #ytshorts
Limestone, Quicklime and Slaked Lime Video Luke Magson
The Limestone Cycle
Limestone vs Water
Комментарии
 0:04:32
0:04:32
 0:04:32
0:04:32
 0:08:33
0:08:33
 0:01:27
0:01:27
 0:04:03
0:04:03
 0:01:31
0:01:31
 0:04:40
0:04:40
 0:03:19
0:03:19
 0:00:11
0:00:11
 0:02:28
0:02:28
 0:02:01
0:02:01
 0:01:07
0:01:07
 0:07:24
0:07:24
 0:02:01
0:02:01
 0:10:15
0:10:15
 0:01:50
0:01:50
 0:00:12
0:00:12
 0:07:08
0:07:08
 0:00:22
0:00:22
 0:00:07
0:00:07
 0:00:14
0:00:14
 0:02:11
0:02:11
 0:05:41
0:05:41
 0:00:12
0:00:12