filmov
tv
Ionic Charge for Lead (Pb)

Показать описание
Lead (Pb) is a transition metal and therefore it can have a different ionic charge depending what it is chemically bonded to. The Pb 2+ and Pb 4+ ions, named Lead (II) and Lead (IV) respectively, are the ions Kead will form.
When bonding with other elements Lead can lose/transfer two electrons and become Pb 2+. If it loses four electrons it will become Pb 4+ .
Note that when we look at the charge on ions, it is important to understand that ions come in pairs. For example, n an ionic compound like NaCl, the Na is a positive ion (Na+) and has transferred/lost an electron to the Chlorine atom which now has an extra electron and is negative (Cl-). We can dissolve NaCl in water and it will split apart into Na+ and Cl-. The electron from the Na is now with the Cl.
----- Helpful Videos -----
Note that ionic charge and oxidation number are different concepts. While there is overlap, they are not the same thing. In this video we are discussing the ionic charge on Lead.
When bonding with other elements Lead can lose/transfer two electrons and become Pb 2+. If it loses four electrons it will become Pb 4+ .
Note that when we look at the charge on ions, it is important to understand that ions come in pairs. For example, n an ionic compound like NaCl, the Na is a positive ion (Na+) and has transferred/lost an electron to the Chlorine atom which now has an extra electron and is negative (Cl-). We can dissolve NaCl in water and it will split apart into Na+ and Cl-. The electron from the Na is now with the Cl.
----- Helpful Videos -----
Note that ionic charge and oxidation number are different concepts. While there is overlap, they are not the same thing. In this video we are discussing the ionic charge on Lead.
Ionic Charge for Lead (Pb)
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions)
Ionic Charge for Silver (Ag)
Write the full electronic configuration of lead Pb
Calligraphy Chemist Presents: electronic configuration of lead
Lead (Pb) Full Electron configuration- AP Chemistry Unit 1 #ap #apchemistry
What is the C Rate for Lead-Acid and Lithium Batteries? How to Calculate
Lead. Lead(pb). SCIENCE KNOWLEDGE.
How to find the Number of Protons, Electrons, Neutrons for Lead (Pb)
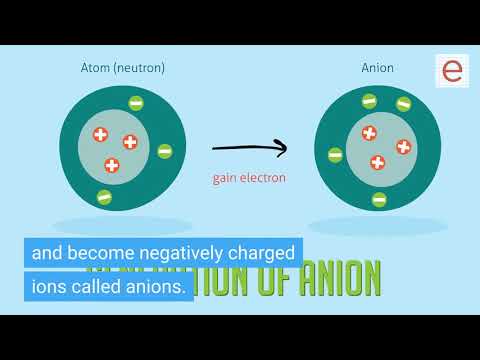
Determining the Charge of Ions
Is Pb(NO3)2, Lead (II) nitrate, Ionic or Covalent?
How to Find the Ionic Charge for Strontium (Sr)
Why is lead Pb? #Shorts
How To Recondition Any ION Old Sealed PB Lead Acid Battery That Won't Hold A Charge
Charges of transition metals, Zn, Ag
Which elements tend to lose electrons? What charge will they become?
Charge old UPS battery (lead acid) with High Voltage experiment
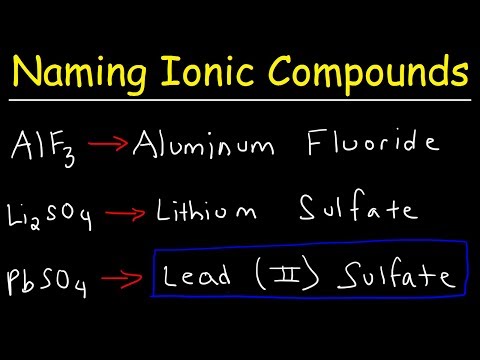
Writing Ionic Formulas with Transition Metals
3.4 - Ions and Charges
How To Name Ionic Compounds With Transition Metals
Variable valency-Lead #rcfychemistry #variablevalency #valency #chemistry #lead #leadvalency
Introduction to Lead (Pb) Isotopes and Decay
How to Write the Name for Pb(SO4)2
How to Find the Ionic Charge for Barium (Ba)
Комментарии
 0:02:33
0:02:33
 0:03:49
0:03:49
 0:02:19
0:02:19
 0:05:42
0:05:42
 0:00:18
0:00:18
 0:00:55
0:00:55
 0:05:38
0:05:38
 0:01:06
0:01:06
 0:03:09
0:03:09
 0:32:32
0:32:32
 0:02:01
0:02:01
 0:01:05
0:01:05
 0:00:21
0:00:21
 0:10:13
0:10:13
 0:04:10
0:04:10
 0:01:34
0:01:34
 0:00:21
0:00:21
 0:07:21
0:07:21
 0:23:20
0:23:20
 0:13:33
0:13:33
 0:00:14
0:00:14
 0:02:22
0:02:22
 0:01:37
0:01:37
 0:01:03
0:01:03