filmov
tv
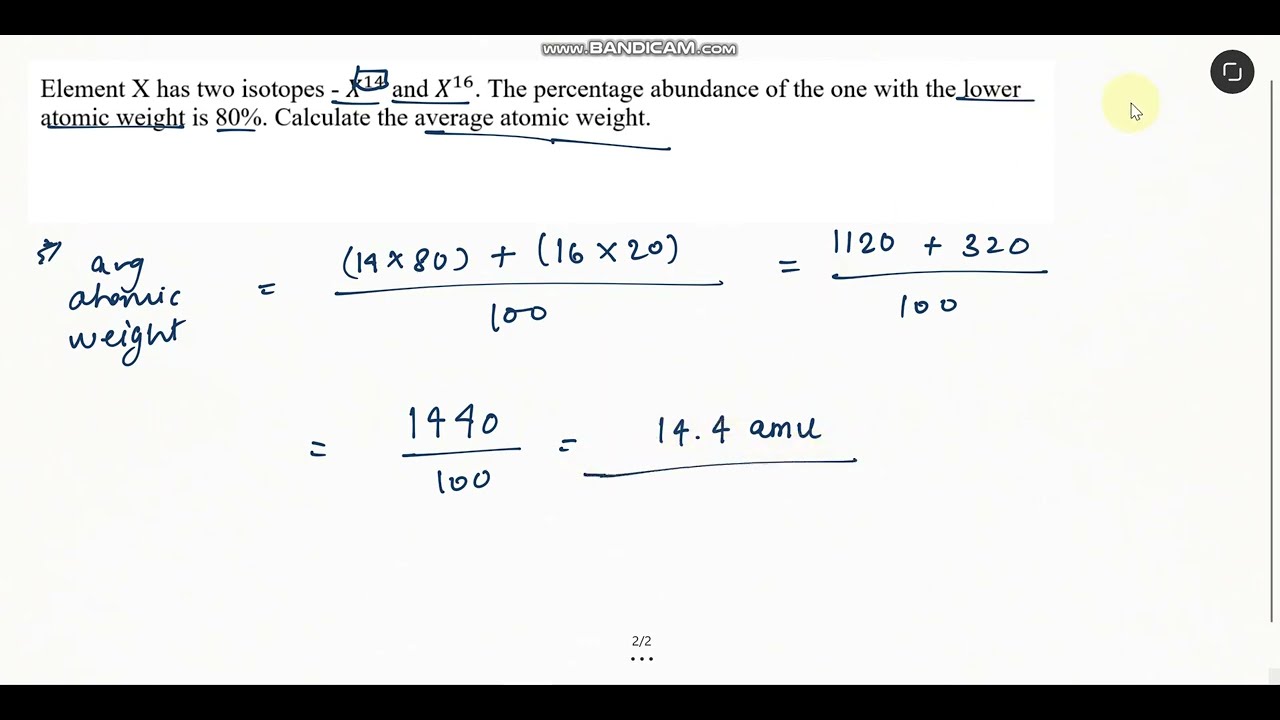
Element X has two isotopes- X14 and X16. The % abundance of one with the lower atomic weight is 80%
Показать описание
Element X has two isotopes - X^14 and X^16. The percentage abundance of the one with the lower atomic weight is 80%. Calculate the average atomic weight.
Answer: 14.4 amu
Answer: 14.4 amu
 0:01:22
0:01:22
 0:03:44
0:03:44
 0:01:50
0:01:50
 0:05:12
0:05:12
 0:11:49
0:11:49
 0:07:19
0:07:19
 0:03:54
0:03:54
 0:10:18
0:10:18
 0:01:49
0:01:49
 0:02:23
0:02:23
 0:07:20
0:07:20
 0:03:26
0:03:26
 0:03:33
0:03:33
 0:01:03
0:01:03
 0:03:49
0:03:49
 0:03:04
0:03:04
 0:04:39
0:04:39
 0:12:42
0:12:42
 0:01:46
0:01:46
 0:04:51
0:04:51
 0:06:32
0:06:32
 0:01:33
0:01:33
 0:07:06
0:07:06
 0:07:23
0:07:23