filmov
tv
Give Basic Theory of UV Spectroscopy. #Spectroscopy #Organic Chemistry

Показать описание
U.V. spectroscopy is based on the electronic excitation of molecules. The absorptions from the ultraviolet regions supply energy which is sufficient to raise an electron form one energy level to a higher one.
According to the molecular orbital approach the absorbed energy of light is used to transfer an electron from a bonding or non-bonding molecular orbital to an anti-bonding molecular orbital.
For example in acetone all single bonds are sigma bond, while double bond is pi bond and lone pairs of electrons with oxygen which don’t take part in bonding are non bonding electrons.
Now, the electron thus transferred may be a sigma (𝛔) bond electron or a pi (𝛑) bond electron or it may be an electron of a lone-pair (n).i.e. a non-bonding pair.
The possible electronic transitions (→) that are involved in ultraviolet and visible region are:
𝛔 → 𝛔*
𝛔 → 𝛑*
𝛑 → 𝛔*
𝛑 → 𝛑*
n → 𝛔*
n → 𝛑*
A 𝛑 → 𝛑* transition occurs with a compound containing double bond and an n → 𝛑* transition occurs with a compound containing heteroatom, e.g. Thus increasing amounts of energy are required to raise n, 𝛑 and 𝛔 electrons from their bonding to the corresponding anti-bonding molecular orbitals. In fact, 𝛔 electrons are held very tightly and hence the energy required for 𝛔 → 𝛔* transition is so much that it requires absorption of wavelengths from the far ultraviolet region.
Other Subjects:
Комментарии
 0:02:37
0:02:37
 0:09:37
0:09:37
 0:00:58
0:00:58
 0:03:31
0:03:31
 0:05:50
0:05:50
 0:13:45
0:13:45
 0:06:48
0:06:48
 0:15:43
0:15:43
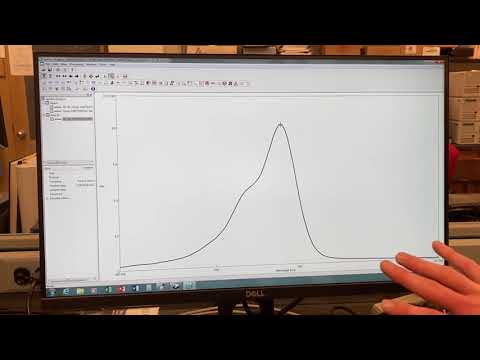 0:02:44
0:02:44
 0:26:18
0:26:18
 0:20:49
0:20:49
 0:04:10
0:04:10
 0:17:05
0:17:05
 0:10:49
0:10:49
 0:08:13
0:08:13
 0:18:25
0:18:25
 0:03:37
0:03:37
 0:29:16
0:29:16
 0:03:54
0:03:54
 0:03:34
0:03:34
 0:26:23
0:26:23
 0:05:33
0:05:33
 0:00:15
0:00:15
 0:01:52
0:01:52