filmov
tv
Explain the principle of Fluorescence and Phosphorescence. | Analytical Chemistry

Показать описание
Many compounds absorb ultraviolet or visible light and undergo an electronic transition from low electronic energy levels to high electronic, energy levels. This Absorption of light requires 10-15 sec.
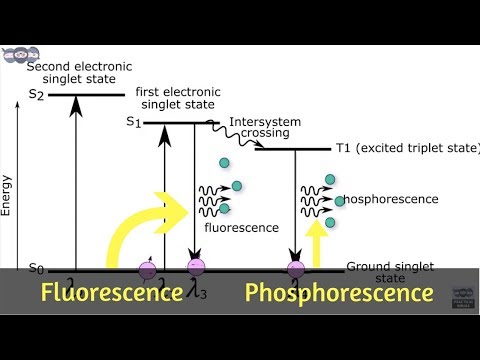
The instant re-emission of the absorbed energy is called Fluorescence.
While delayed re-emission of the absorbed energy is called Phosphorescence.
For most molecules, the electrons are paired in the ground state i.e. pair of electrons have opposite spins this is called singlet state. As the spin is paired up, they do not show any magnetic moment.
But if the electrons have the same spin the magnetic moment of both the electrons gets combined and they behave like a tiny magnet. This state where the electron pair is having the same spin is called as triplet state.
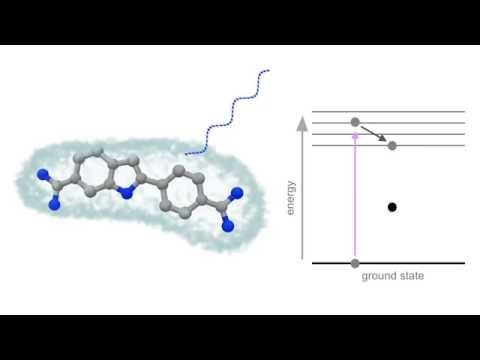
In a molecule, there are several electronic energy levels and electrons are present at the ground state in the singlet state i.e. with opposite spins. When a molecule absorbs U.V. or Visible light, one electron from ground state undergoes to an excited state. Now the excited state is also having several vibrational levels, so the excited electron loses its energy by intermolecular collision and it comes to the lowest vibrational level of the excited state. Finally, the electron from the lowest vibrational level of excited state returns to the ground state by emitting the radiation or light of lower energy or higher wavelength.
This overall process of absorption, intermolecular collision, and emission take 10-4 to 10-8 sec which is very small thus it looks like instant re-emission of light. But the energy of radiation emitted is always less than the energy of radiation absorbed. Or in other words, the wavelength of emitted radiation will be always higher than the wavelength of radiation absorbed. This is Fluorescence phenomenon.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
The instant re-emission of the absorbed energy is called Fluorescence.
While delayed re-emission of the absorbed energy is called Phosphorescence.
For most molecules, the electrons are paired in the ground state i.e. pair of electrons have opposite spins this is called singlet state. As the spin is paired up, they do not show any magnetic moment.
But if the electrons have the same spin the magnetic moment of both the electrons gets combined and they behave like a tiny magnet. This state where the electron pair is having the same spin is called as triplet state.
In a molecule, there are several electronic energy levels and electrons are present at the ground state in the singlet state i.e. with opposite spins. When a molecule absorbs U.V. or Visible light, one electron from ground state undergoes to an excited state. Now the excited state is also having several vibrational levels, so the excited electron loses its energy by intermolecular collision and it comes to the lowest vibrational level of the excited state. Finally, the electron from the lowest vibrational level of excited state returns to the ground state by emitting the radiation or light of lower energy or higher wavelength.
This overall process of absorption, intermolecular collision, and emission take 10-4 to 10-8 sec which is very small thus it looks like instant re-emission of light. But the energy of radiation emitted is always less than the energy of radiation absorbed. Or in other words, the wavelength of emitted radiation will be always higher than the wavelength of radiation absorbed. This is Fluorescence phenomenon.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
Комментарии
 0:03:54
0:03:54
 0:02:26
0:02:26
 0:02:05
0:02:05
 0:08:02
0:08:02
 0:04:38
0:04:38
 0:03:40
0:03:40
 0:04:13
0:04:13
 0:13:21
0:13:21
 0:02:19
0:02:19
 0:17:03
0:17:03
 0:00:58
0:00:58
 0:02:01
0:02:01
 0:02:51
0:02:51
 0:05:42
0:05:42
 0:08:01
0:08:01
 0:50:38
0:50:38
 0:01:58
0:01:58
 0:04:18
0:04:18
 0:01:28
0:01:28
 0:14:54
0:14:54
 0:07:05
0:07:05
 0:01:52
0:01:52
 0:06:27
0:06:27
 0:15:02
0:15:02